Chromatography covers a broad range of physical methods used to separate and/or analyse complex mixtures.
The constituents to be separated are distributed between two phases: a stationary phase and a mobile phase, which percolates through the stationary phase.
A mixture is introduced into one end of the stationary phase, which is contained in a column or coated onto a substrate and the contents are flushed through the system.
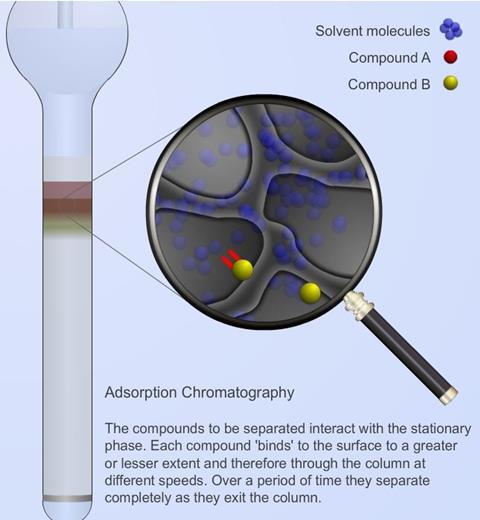
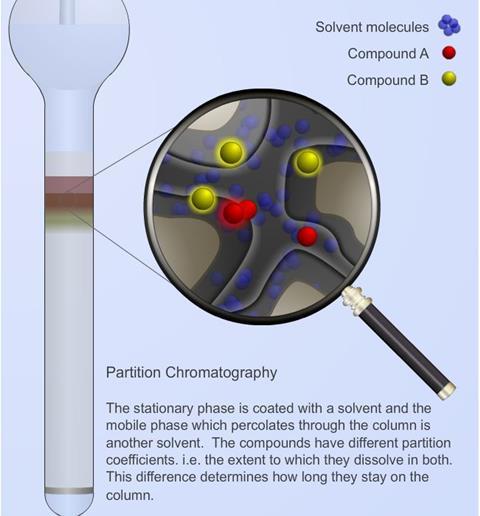
Each constituent is adsorbed (or dissolved) to a greater or lesser extent as passage through the stationary phase takes place and since each therefore migrates at a different rate, separation of the mixture is achieved.
A simple example is the separation of inks using paper chromatography.
Interested in paper chromatography?
See examples and find links to various investigations here.
Chromatography has developed into a highly sophisticated and varied procedure which is used in chemical or bio-processing industries; the need to separate and purify a product from a complex mixture is a very necessary and highly important step in the production line. The separation can be achieved with great precision; even very similar compounds, such as proteins that may only vary by a single amino acid, can be separated this way. In fact, chromatography can purify any soluble or volatile substance if the right adsorbent material, carrier material, and operating conditions are employed.
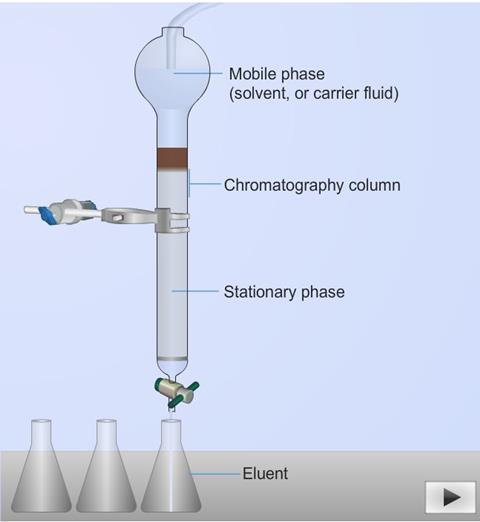
Although there are other types of chromatography e.g. paper chromatography and thin layer chromatography (TLC), most modern applications of chromatography employ a column. The column is where the actual separation takes place. It is usually a glass or metal tube of sufficient strength to withstand the pressures that may be applied across it. The column contains the stationary phase. The mobile phase runs through the column and is adsorbed onto the stationary phase. The column can either be a packed bed or open tubular column.
The basics – the mobile and stationary phases
The mobile phase is comprised of a solvent into which the sample is injected. The solvent and sample flow through the column together; thus the mobile phase is often referred to as the “carrier fluid.” The stationary phase is the material in the column for which the components to be separated have varying affinities. The materials which comprise the mobile and stationary phases vary depending on the general type of chromatographic process being performed. The mobile phase in gas chromatography is generally an inert gas. The stationary phase is generally an adsorbent solid or liquid distributed over the surface of a porous, inert support.
Liquid Chromatography
- The mobile phase in liquid chromatography is a liquid of low viscosity which flows through the stationary phase bed. This bed is usually an inert solid such as silica gel (SiO2.xH2 O) or alumina (Al2 O3.xH2 O).
- The effectiveness of the separation is controlled by temperature since higher temperatures improve the adsorbing properties of the stationary phase.
- A wide range of solvents can be used in liquid chromatography and the optimum solvent is often chosen by running preliminary TLC experiments.
- Liquid chromatography can be improved by applying pressure above the liquid in the column; nitrogen is often used at a pressure of approximately 2 atm.
Adsorption chromatography
Partition chromatography
Downloads
A liquid chromatography column
Simulation | Flash, Size 19.19 kbAdsorption chromatography
Simulation | Flash, Size 44.53 kbPartition chromatography
Simulation | Flash, Size 32.78 kb
Chromatography

Chromatography covers a broad range of physical methods used to separate and/or analyse complex mixtures. It can be preparative or analytical and has a wide range of applications.
 Currently
reading
Currently
reading
Introduction
- 2





















1 Reader's comment