Rig yourself a polymerisation station and show students how to form nylon in this practical demonstration
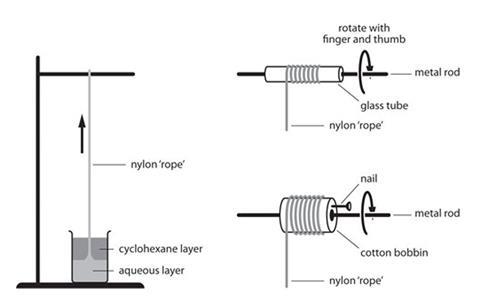
Perform this ‘trick’ with a solution of decanedioyl dichloride in cyclohexane floated on an aqueous solution of 1,6-diaminohexane. As nylon forms at the interface, it can be pulled out as fast as it is produced, forming a long thread: the ‘nylon rope’.
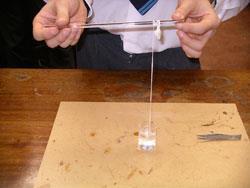
Equipment
Apparatus
- Eye protection
- Beaker, 25 cm3
- A pair of tweezers
- Retort stand
- Boss and clamp
Chemicals
- 1,6-diaminohexane (hexamethylene diamine, hexane-1,6-diamine, H2N(CH2)6(NH2), 2.2 g (HARMFUL, CORROSIVE)
- Decanedioyl dichloride (sebacoyl chloride, ClOC(CH2)8COCl), 1.5 g (HARMFUL, IRRITANT, CAUSES SEVERE BURNS AND EYE DAMAGE)
- Cyclohexane, 50 cm3 (IRRITANT, HIGHLY FLAMABLE,)
- Deionised water, 50 cm3
Health, safety and technical notes
- Read our standard health and safety guidance. CLEAPSS members should consult PP042.
- Always wear splash-proof goggles.
- Avoid skin contact.
- Work in a well-ventilated room away from sources of ignition.
- 1,6-diaminohexane (hexamethylene diamine, hexane-1,6-diamine, H2N(CH2)6(NH2) is corrosive, harmful if swallowed or in contact with skin and a respiratory irritant (see CLEAPSS Hazcard HC003b).
- Decanedioyl dichloride (sebacoyl chloride, ClOC(CH2)8COCl) is corrsive, causes severe burns and eye damage, harmful if swallowed (see CLEAPSS Hazcard HC041).
- Cyclohexane is highly flammable, and a skin/respiratory irritant (see CLEAPSS Hazcard HC045b).
Disposal
Dispose of the mixture as follows:
- Wear splash-proof goggles. Avoid skin contact.
- Use a fume cupboard.
- In a large beaker, stir together the unused solutions plus the used mixture. A lump of nylon will be produced which can be removed with tweezers, rinsed well with water, and disposed as solid waste.
- The remaining liquids should be separated using a separating funnel. The lower (aqueous) layer can be flushed down the foul-water drain with plenty of water. The upper (organic) layer should be transferred to a hydrocarbon waste bottle and stored in a flammable cabinet until the next hazardous waste collection.
- Failure to do this may result in the polymerisation taking place in the sink, leading to a blockage.
Procedure
Work in a well-ventilated laboratory. Wear eye protection and disposable nitrile gloves when pulling out the thread.
Before the demonstration
- Make up a solution of 2.2 g of 1,6-diaminohexane in 50 cm3 of deionised water. This solution is approximately 0.4 mol dm-3.
- Make up a solution of 1.5 g of decanedioyl dichloride in 50 cm3 of cyclohexane. This solution is approximately 0.15 mol dm–3.
The demonstration
- Pour 5 cm3 of the aqueous diamine solution into a 25 cm3 beaker. Carefully pour 5 cm3 of the cyclohexane solution of the acid chloride on top of the first solution so that mixing is minimised. Do this by pouring the second solution down the wall of the beaker, or pour it down a glass rod.
- The cyclohexane will float on top of the water without mixing.
- Place the beaker below a stand and clamp as shown (see below). A greyish film of nylon will form at the interface.
- Pick up a little of this with a pair of tweezers and lift it slowly and gently from the beaker. It should draw up behind it a thread of nylon.
- Pull this over the rod of the clamp so that this acts as a pulley.
- Continue pulling the nylon thread at a rate of about half a metre per second. It should be possible to pull out several metres.
- Take care, the thread will be coated with unreacted monomer and may in fact be a narrow, hollow tube filled with monomer solution. Wearing disposable gloves is essential.
Notes
- The beaker is rather small so allow the audience as close as possible consistent with comfort and safety.
- Point out that this demonstration is different from the industrial method of making nylon, which takes place at a higher temperature. Molten nylon is then forced through multi-holed ‘spinnerets’ to form the fibres.
- The reaction is a condensation polymerisation:
- nH2N(CH2)6 NH2 + nClOC(CH2)8COCl → H2N[(CH2)6NHCO(CH2)8]nCOCl + nHCl
- The nylon formed is nylon 6 –10 so called because of the lengths of the carbon chains of the monomers.
- Nylon 6 – 6 can be made using hexanedioyl dichloride (adipoyl chloride). The diamine is present in excess to react with the hydrogen chloride that is eliminated. An alternative procedure is to use the stoichiometric quantity of diamine dissolved in excess sodium hydroxide solution.
Downloads
Making nylon - the ‘nylon rope trick’ - teacher notes
PDF, Size 0.18 mb
Additional information
This practical is part of our Chemistry for non-specialists and Classic chemistry demonstrations collections. This experiment has been adapted from Classic Chemistry Demonstrations, Royal Society of Chemistry, London, p.159-161.


















No comments yet