The periodicity and reactions of the period three elements appear in many post-16 chemistry courses. You can show most of these elements and their reaction with oxygen to students reasonably easily, but getting hold of samples has become very tricky. However, Melissa Golden and colleagues have shown that you can generate a small sample of white phosphorus from the readily available red allotrope and react the phosphorus in situ with oxygen.1
Kit
- Glass Pasteur pipette
- 50 cm3 gas syringe with silicone tubing to fit wide opening of pipette2
- Dry red phosphorus (flammable, CLEAPSS Hazcard 73B)
- Glass wool
- Ethanol spirit burner
- Universal indicator solution (highly flammable, CLEAPSS Hazcards 32 and 47, recipe book 47)
- Watch glass
- A micro-spatula or wooden splint
Preparation
Wear gloves and eye protection while working with the glass wool. Use a micro-spatula or wooden splint to plug the nozzle of the Pasteur pipette with a small piece of glass wool. Holding the pipette horizontally, place a micro-spatula of dry red phosphorus into the pipette 1–2 cm away from the plug. Then plug the pipette tube in a similar fashion 1–2 cm further up the pipette. The phosphorus should be trapped between the two wool plugs with an air gap of at least 1 cm either side.
Finally, clamp a gas syringe horizontally and fit the syringe to the wide end of the pipette with a piece of silicone tubing. Check you can draw air in and out of the pipette easily by slowly moving the piston of the syringe. The glass wool should act only to prevent solids from passing out into the nozzle of the pipette (and to reduce the speed oxygen from the air diffuses in during the demonstration). Leave the syringe with the piston in the withdrawn position, ready to pass air through during the demonstration. The weight of the pipette will cause it to droop so support the narrow end by resting it in a second clamp.
In front of the class
Work in a well-ventilated area. The audience should wear eye protection. Dim the lights slightly to get the best effect from the flame. Ensure the nozzle of the pipette points away from audience members and flammable materials. Light the spirit burner and use it to heat the red phosphorus in the pipette.
After a short time, the flame colour will turn more orange, indicating the presence of sodium in the flame from the glass – the pipette will soften at this point. At approximately the same time, you will see white vapours inside the tube (a combination of phosphorus oxides and white phosphorus which remains once the oxygen in the pipette has been consumed). Also point out to students the cream/yellow colour depositing on the glass wool which they may mistake for the pyrolysis of cotton wool.
Extinguish and remove the spirit burner. Then expel the white phosphorus by squeezing the syringe. When it contacts oxygen in the air at the end of the nozzle, the white phosphorous spontaneously combusts to give an intense white/orange flame and a puff of white smoke.
Once the flame has gone out and the pipette has cooled, you can once more blow air through it with the syringe, this time with the end submerged in a watchglass containing water and universal indicator solution. The solution will turn red, showing the presence of phosphoric acid.
Teaching goal
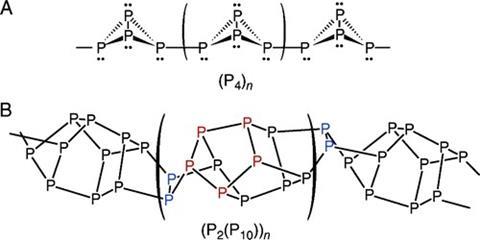
The red allotrope of phosphorus is an amorphous polymer. It is significantly more stable at room temperature than the white form, which consists of tetrahedra of P4 with each atom bonded to three others with a lone pair facing outwards. Valence shell electron pair repulsion (VSEPR) theory predicts bond angles of about 107°, but the shape of the molecule necessitates highly strained bonds closer to 60°. This explains the high reactivity of the white allotrope.
The red form has traditionally been thought to consist of P4 tetrahedra connected end-to-end in polymeric chains. However, more recent research suggests a more amorphous structure, consisting primarily of (P2(P10)) repeating units.
The red allotrope sublimes at about 450˚C. Above 900˚C an equilibrium begins to establish between the P4 and P2 allotropes with the diatomic form becoming more favourable at higher temperatures. Although students seeing this demonstration may not have been introduced to the concept of entropy, it’s worth noting this is in line with a simple prediction based on the number of particles present in the system increasing as the size of each molecule decreases – the positive change in entropy of the system becomes more important at higher temperatures.
P(red) ⇌ P4(white) ΔH +17.6 kJ mol-1
The oxidation and hydrolysis reactions of phosphorus were discussed in the November 2015 Exhibition Chemistry article – the Phosphorous Sun (rsc.li/2nc9UQF).
The oxidation and hydrolysis reactions of phosphorus were discussed in the Phosphorous Sun demonstration.
Safety and disposal
Do not handle glass wool with bare hands. Do not attempt to dry the phosphorus if it looks damp.
Any remaining white phosphorus can be removed by leaving the pipette in 0.2–0.5 M copper sulfate solution. The pipette can then be rinsed through with water and the washings disposed of down the sink with plenty of water. The glass wool and pipette can be disposed of in the glass bin.
Downloads
Technician notes
Word, Size 54.38 kbTechnician notes
PDF, Size 49.34 kb
References
1. M L Golden, J. Chem. Educ., 2010, 87, 1154 (DOI: 10.1021/ed1002652)
2. Credit for this idea goes to Bob Worley at CLEAPSS. The original paper described using a large pipette bulb but a gas syringe gives you a larger quantity of air at your disposal and more control over its flow rate.
3. Reprinted with permission from J. Chem. Educ., 2010, 87, 1154. Copyright 2010 American Chemical Society.


















No comments yet