‘Whoa! Can you do that again?’ a pupil asked, reaching for their phone to film the moment for posterity. With a little bit of preparation, you can easily reproduce this student-pleasing effect by treating a regular piece of filter paper with a nitrating mixture, turning it into … flash paper.
Kit
- 20 cm3 concentrated sulfuric(VI) acid (corrosive)
- 20 cm3 concentrated nitric(V) acid (corrosive, oxidising)
- Approx 55 g sodium carbonate (irritant)
- Piece of filter paper (approx 1 g)
- Gratnells tray (or similar sized tray of at least 5 cm depth)
- Glass stirring rod
- Forceps
- Polypropylene microwaveable food dish, or ice cream tub, preferably circular with max 25 cm diameter* (look for recycling symbols PP or 05)
* Ideal dishes for this activity will be more than 1 cm deep and have handles on the sides for a good grip. If the dish is any larger, you won’t be able to cover the base of the tub/tray with the nitrating mixture.
Preparation
Wear splash-proof goggles (or a face shield) and chemical-resistant gloves when creating and using the nitration mixture. Work in a well-ventilated laboratory and avoid inhaling any vapours.
Add approx 55 g of sodium carbonate to a Gratnells tray, and fill with water to approx 3 cm to act as a heat sink for the reaction, and to neutralise unreacted acid before disposal. Float the disposable dish in the water and add the acids, taking care when you do.
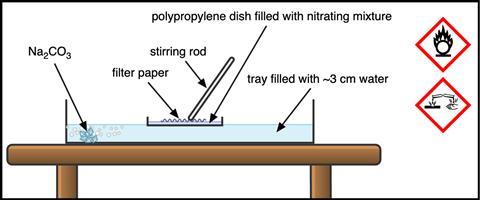
Add the filter paper and use the glass stirring rod to agitate, ensuring there is good coverage of the nitrating mixture (which will average less than 1 mm in depth in a dish of this size). Leave the filter paper for 15 minutes before removing with forceps and dropping straight into the surrounding sodium carbonate solution. Use the forceps to agitate the paper until all fizzing has stopped. Press the neutralised paper between two other sheets of kitchen or lab roll to remove excess moisture and leave to dry thoroughly.
Dispose of the nitrating mixture immediately by carefully pouring any remaining acid into the sodium carbonate solution in the surrounding tray. The now-neutralised solution can be poured down a foul water drain.
In front of the class

First show students how an untreated piece of filter paper burns to emphasise the reliance on oxygen diffusing in from the air to sustain the combustion reaction. Then put pieces of the flash paper on a wire gauze placed on a tripod over a heat-resistant mat and ignite with a lit splint. You can safely light small pieces (up to ~2 cm) using a pair of tweezers and holding them at arm’s length. The paper will burn immediately with a bright yellow flame (thanks in part to the sodium carbonate bath), leaving almost no residual material.
Safety and disposal notes
- Ensure an experienced chemistry teacher prepares and uses the nitrating mixture.
- It’s unlikely that this demonstration is covered in model risk assessments.
- Members can obtain a special risk assessment from CLEAPSS.
- Do not exceed the quantities outlined above or deviate from the method.
- Avoid using fuming nitric acid to prepare nitrocellulose because this could produce more than 12.6% nitrogen, for which UK schools would need an explosives certificate.
- Safely dispose of unused flash paper; it should be created fresh. The paper decomposes over time and other staff members could mistake this for ordinary paper.
Teaching goal
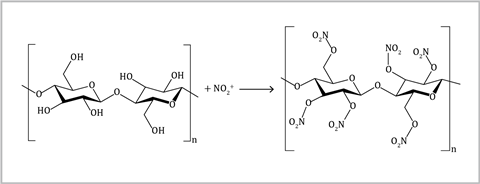
Nitrocellulose is used as a propellant and has been popular with magicians since the 19th century. The demonstration is ideally suited to lessons on combustion with younger students, where you can show them the impact of available oxygen levels. The demonstration could also complement teaching nitration reactions to post-16 students. Mechanistic studies have shown that the reaction follows an analogous pathway similar to that of the nitration of benzene, where the nitronium ion is produced in the acid mixture, and then substitutes the hydrogen atom of a hydroxyl group on the cellulose.
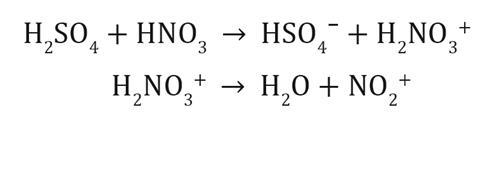
Downloads
The production and combustion of nitrocellulose technician notes
PDF, Size 0.17 mbThe production and combustion of nitrocellulose technician notes
Word, Size 0.49 mb

















No comments yet