Use this class practical to reveal how methanol and ethanol behave differently when treated with iodine and sodium hydroxide solutions. These two important alcohols may be chemically distinguished by using the iodoform reaction
This experiment takes about 20 minutes. It is eminently suitable for students who have the iodoform reaction in their specification. For others, it is a good observational exercise and an extension for more able students.
Both methanol and ethanol (and other alcohols and organic liquids) can be stored in plastic pipettes, from which they can be easily dispensed. The main difficulty for students arises from the fact that if the pipettes are squeezed too hard, the alcohols come out of the pipette in a stream (because of their low surface tension). Students must handle the pipettes very carefully, and some practice will be required before proceeding with this experiment.
Equipment
Apparatus
- Eye protection (goggles)
- Test tubes x2 for each group of students
Chemicals
- Liquids or solutions in plastic dropping pipettes (one of each):
- Methanol (HIGHLY FLAMMABLE, TOXIC)
- Ethanol (HIGHLY FLAMMABLE) or Industrial denatured alcohol, IDA (HIGHLY FLAMMABLE, HARMFUL)
- Sodium hydroxide, 1 M (CORROSIVE)
- Iodine solution, 0.5 M (dissolved in potassium iodide solution 0.2 M)
Health, safety and technical notes
- Read our standard health and safety guidance
- Wear goggles throughout.
- Methanol, CH3OH(l), (HIGHLY FLAMMABLE, TOXIC) – see CLEAPSS Hazcard HC040b.
- Ethanol, CH3CH2OH(l), (HIGHLY FLAMMABLE) and IDA (HIGHLY FLAMMABLE, HARMFUL) – see CLEAPSS Hazcard HC040a.
- Sodium hydroxide, NaOH(aq), (CORROSIVE at concentration used) – see CLEAPSS Hazcard HC091a and CLEAPSS Recipe Book RB085.
- Iodine solution, I2(aq), dissolved in potassium iodide solution, KI(aq), – see CLEAPSS Hazcard HC054 and CLEAPSS Recipe Book RB050.
- Product: Tri-iodo-methane, CHI3(s), (HARMFUL) – see CLEAPSS Hazcard HC104.
Procedure
- Practice (over a sink) producing single drops of ethanol from the pipette.
- Add 10 drops of methanol to one test tube.
- Add 10 drops of ethanol to the other test tube.
- Add 25 drops of iodine solution to each alcohol.
- Add 10 drops of sodium hydroxide solution to each alcohol.
- Gently swirl the test tubes a few times. The dark colour of the iodine should start to fade.
- After two minutes carefully observe the two test tubes. What differences do you notice?
Teaching notes
The solution in the ethanol test tube should go cloudy and then a yellow precipitate of tri-iodo-methane (iodoform) should be seen. This has a distinct ‘antiseptic’ smell. The methanol test tube should remain clear.
The iodoform reaction is given by compounds with a methyl group next to a carbonyl group. Secondary alcohols with a CH3 on the carbon carrying the OH (eg propan-2-ol) that can be oxidised to carbonyl compounds of this type, also give a positive iodoform test. (NB carboxylic acids do not)
Ethanol is the only primary alcohol which will give the reaction and ethanal the only aldehyde. An explanation of the reaction is:
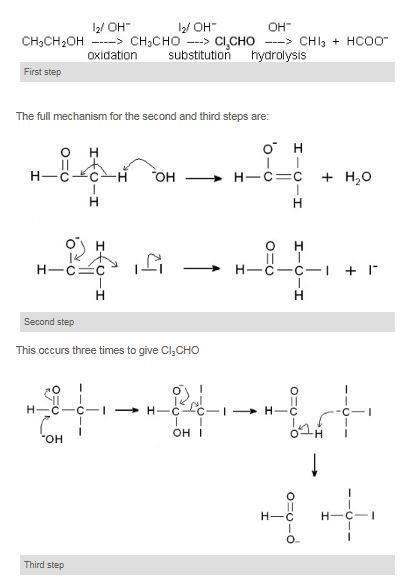
The negative charge on the carbon in the I3C– ion is stabilised by the three electronegative iodine atoms.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet