Gas jars, acid, and a little copper come together to create a diffusion practical that will explore bromine and its alternatives
Bromine can be a dangerous chemical to use in a classroom, and this practical gives a safer alternative to bromine in your learning space.
Equipment
Apparatus
- Eye protection
- Gloves
- Measuring cylinder, 250 ml
- Retort stand
- Boss and clamp
- Gas jar x 3
- Gas jar lid
- Fume cupboard
Chemicals
- Bromine
- Nitrogen dioxide
- Nitric acid
- Water
- Copper turnings, 2 g
Health, safety and technical notes
- Read our standard health and safety guidance.
- Always wear eye protection
- Wear gloves.
- Bromine is toxic and corrosive (see CLEAPSS Hazcard HC015b).
- Nitrogen dioxide is toxic and corrosive (see CLEAPSS Hazcard HC068b).
- Nitric acid is corrosive (see CLEAPSS Hazcard HC067).
- Copper turnings are of low hazard (see CLEAPSS Hazcard HC062).
Procedure
- Using water and a 250 ml measuring cylinder, establish the volume of the gas jar. Do not use this wet gas jar for the following demonstration.
- Using a retort stand, boss and clamp, adjust the fitting of a dry inverted gas jar over another dry gas jar of the same size and set it to one side.
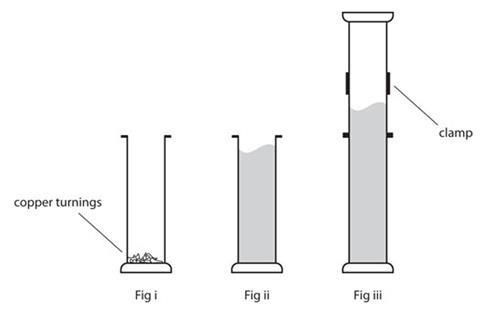
- In a fume cupboard, place at least 1 g, but no more than 2 g, of copper turnings in the gas jar (fig i).
- Knowing that 8 ml of concentrated nitric acid produces 1000 cm3 of nitrogen dioxide at room temperature and pressure, estimate the volume of acid needed to just fill the gas jar with gas.
- Wearing eye protection and suitable gloves, place 1 ml less than the estimated volume of nitric acid in a 10 ml measuring cylinder. Empty the contents of the measuring cylinder into the gas jar with copper and watch the brown gas rise (fig ii).
- Once the reaction stops, it is possible to place a lid on the gas jar and carry out the rest of the demonstration in the open laboratory.
- Invert the second jar over the jar containing the gas and remove the lid from the lower jar (containing the nitrogen dioxide)
- Clamp this jar into position with care (fig iii).
- Diffusion takes place in 20 minutes.
Notes
- Wear goggles (EN 166 3) when handling concentrated nitric acid. Gloves are not required when an automatic pipettor is used.
- If possible, move the gas jars to a fume cupboard. Add water to each gas jar and pour the contents down a foul-water drain, adding more water. Unreacted copper turnings can be dried and reused. If there is no fume cupboard in the room, carefully insert gas-jar lids to cover both jars. Seal with sellotape and remove to a fume cupboard.
- The demonstration can be performed along with a similar set-up using bromine to show that gases diffuse at different rates. To fill a 1 litre gas jar, use no more than 2 ml of liquid bromine. Adjust the volume of bromine liquid to the capacity of the gas jar that is available. It takes time for bromine to vaporise. Use a fume cupboard, wear goggles or a face shield and nitrile or latex chemical-resistant gloves. A bucket of 1 M sodium thiosulfate solution should be available in case bromine splashes onto the skin or is spilled.
Downloads
Diffusion of gases – a safer alternative to bromine - teacher notes
PDF, Size 0.17 mb
Additional information
This practical is part of our Chemistry for non-specialists collection. This experiment has been reproduced from CLEAPSS®, L195 Safer chemicals, safer reactions, p.28 with permission from CLEAPSS®.





























No comments yet