Find out what apparatus you need for common microscale experiments, learn about key techniques and discover how to prepare solutions of different elements
Microscale experiments give students the opportunity to develop their observational and investigative skills using simple equipment and smaller quantities of chemicals.
This practical guide is designed to accompany our Microscale chemistry resources, supporting you to use microscale experiments in your classroom. Find out about required apparatus, and discover guidance on techniques such as filtration and titration.
You can also learn how to prepare solutions using compounds of a variety of elements, from calcium to tungsten.
What’s on this page
1. Common microscale apparatus
1.1. Transparent plastic sheet (OHP sheet)
These sheets are widely used in schools and colleges and are used to overlie the student worksheets in several experiments. Students add drops of solutions onto the sheets to do the reactions. The sheets are reasonably resilient and may be wiped clean with household tissues and re-used many times. However, they could be attacked by strong acids and they are stained by iodine solution if left in contact for more than a few minutes.
Alternatively, clear plastic wallets (A4 size) can be used or the student worksheets could be laminated. Again the wallets or laminated sheets can be wiped clean after use.
Whichever type is used, aqueous solutions form nicely-defined drops on the surface which enable chemical reactions to be conveniently carried out. A discussion of the shape of the drops can provide students with interesting insights into the effects of hydrogen bonding on surface tension.
1.2. Plastic pipettes
These very versatile pieces of plastic apparatus may be used for storing solutions and dispensing drops of solution during experiments. By cutting and reshaping them it is possible to make scoops and spatulas, filter funnels, mini reaction vessels and electrolysis apparatus. The two most useful forms of pipette are shown below.
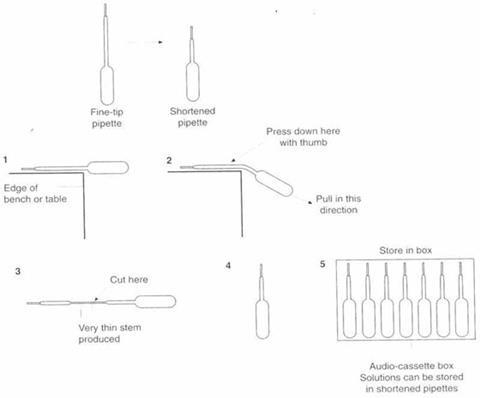
These plastic pipettes have many uses. Two of the most useful are preparing a shortened pipette for storing solutions and making a scoop.
Preparing a shortened pipette
The diagram below shows how to make a shortened pipette from a fine-tip pipette using the edge of a bench and some scissors.
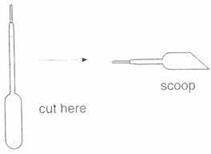
Making a scoop
You can make a scoop from a plastic pipette by cutting the bulb of the pipette at an angle, as in the diagram below.
1.3. Plastic Petri dishes
These are used as containers in which test gases can be generated. Examples are the 4.5 cm and 9 cm diameter Petri dishes supplied by Philip Harris (ref: B8H64838).
1.4. Well-plates
Clear plastic well-plates are sometimes listed in catalogues under culture apparatus. The most useful version is the 24-well plate which consists of an arrangement of 6 × 4 cylindrical wells each well having a capacity of approximately 3 cm3.
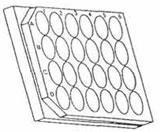
These well-plates are used for organic chemistry experiments, equilibria, rates of reaction and colorimetry experiments. They are compatible with many organic liquids.
1.5. Other apparatus
Other useful pieces of equipment are audio-cassette boxes for storing shortened plastic pipettes, plastic boxes for 35 mm film, plastic display boxes for pens, temperature strips and screw-top sample bottles.
2. Microscale techniques
2.1. Filtration
The diagram below illustrates a simple but effective method for constructing a filter funnel from a plastic pipette.

Your filter funnel is now ready to use. The efficiency of the filter funnel depends mainly on how compact the cotton wool is in the funnel. For coarse particles the cotton wool need not be packed very tightly. However, if very fine particles are to be separated from the liquid tight packing is essential for effective separation.
Note
In microscale filtration, transfer of liquids is always by pipette never by pouring.
2.2. Sampling a bottle of hydroxybenzene (phenol)
Hydroxybenzene is a hazardous substance and sampling a bottle of hydroxybenzene using a spatula is usually difficult. The need to ensure that crystals of hydroxybenzene do not come into contact with the skin, the wearing of gloves and the fact that hydroxybenzene is hygroscopic, causing the crystals to stick together, all add to the difficulties. The following procedure reduces safety hazards and allows students to gain in confidence and practical skills. Students must still wear eye protection and gloves.
This technique illustrates the use of two plastic pipettes for obtaining small samples of hydroxybenzene crystals suitable for use in microscale chemistry experiments.
Procedure
- Take a standard form plastic pipette and cut off the ends as indicated.
![]()
- Cut the tip off the end of a fine-tip pipette as shown below.
![]()
- Taking the modified standard pipette, press it gently down into the crystals in the hydroxybenzene bottle and withdraw. A small column of solid hydroxybenzene should be held on the inside of the bevelled end.
- Place the pipette over the petri dish and insert the fine-tipped pipette to press out a small quantity of hydroxybenzene crystals. (Repeat if necessary at other locations in the petri dish for example if you are investigating the reactions of hydroxybenzene.)
- Place the ends of both pipettes into a 100 cm3 beaker about half full with 1 mol dm−3 sodium hydroxide solution. This dissolves any solid hydroxybenzene remaining on the pipettes.
- The pipettes may then be washed with deionised water, dried on tissue paper and stored ready for reuse.
2.3. Titration
Titrations are widely used in post-16 chemistry courses and also feature in some secondary courses. The traditional apparatus comprises 50 cm3 burettes and 25 cm3 pipettes. For a whole class, experiments with this size of apparatus consume large quantities of solutions and students often take a long time to do the titrations. They are frequently very messy with spillages from pouring solutions and leaking taps on burettes!
Our Microscale chemistry collection offers two microscale approaches for titrations. The simplest method replaces the burette with a pipette. Another set up uses a plastic syringe in place of the burrette. The main advantages of microscale titrations are:
- greatly reduced volumes of solutions required (with the associated reductions in cost)
- removing the need for pouring solutions
- increased speed of titration
- smaller quantities of solutions to dispose of at the end of the experiment
- reduced cognitive load.
Pipette method
The 50 cm3 burette is replaced by a pipette. For a gravimetric titration measure the mass of the reaction vessel before and after, for a volumetric titration the volume is measured by counting the drops of solution added. To do the titration the clamp is carefully tightened on the pipette to add one drop of solution at a time.
Apparatus
- Beaker 10 cm3 or small vial
- White tile
- Clamp stand
- Plastic pipette (preferably a fine-tipped pipette)
- Measuring cylinder, 10 cm3
- Mass balance (to 0.01 g) – for gravimetric titrations
This set up is used to analyse vinegar in Microscale titration.
Syringe method
In this method the 50 cm3 burette is replaced by a 2 cm3 graduated pipette and the 25 cm3 pipette by a 1 cm3 pipette. A diagram of the apparatus is shown below.
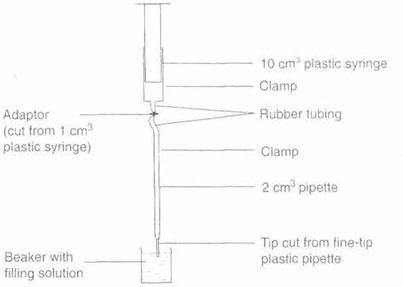
To do the titration the plunger is gently pressed down. The volume of the drops produced is approximately 0.02 cm3 – without the fine tip the drop volume is approximately 0.04 cm3. The apparatus can be read to 0.01 cm3 (compared with 0.05 cm3 with a conventional burette) although the volume of solution delivered (less than 2 cm3) is far less.
The apparatus can be used for the following experiments:
- Measuring an equilibrium constant
- Finding out how much salt there is in seawater
- Measuring the amount of vitamin C in fruit drinks
Apparatus
- Clamp stand with two bosses and clamps
- Graduated pipette, 2 cm3
- Plastic pipette (fine-tip form)
- Plastic syringe, 10 cm3
- Rubber tubing (for connectors)
- Adaptor (made from a 1 cm3 plastic syringe)
- Beaker, 10 cm3
Constructing the burette
- Place the 2 cm3 pipette in a clamp. This is your ‘burette’ during the titration.
- Cut the end off a fine-tip plastic pipette and push it carefully onto the end of the ‘burette’.
- Attach the 10 cm3 plastic syringe to a clamp above the ‘burette’.
- Cut the end off a 1 cm3 plastic syringe to make an adaptor.
- Cut two short pieces of rubber tubing and use them to attach the syringe to the top of the ‘burette’ via the adaptor.
Filling the burette
- Take a 10 cm3 beaker and using a plastic pipette half-fill it with the solution for the ‘burette’.
- Place the tip of the ‘burette’ in the solution and slowly raise the plunger. The solution is drawn up into the ‘burette’. (If air bubbles are drawn up raise and lower the plunger slowly a few times to expel them). When the desired level is reached release the plunger and the liquid level in the ‘burette’ should remain stationary. If the level falls, adjust the connections on the apparatus to ensure that the system is air-tight and repeat the filling process.
2.4. Constructing a conductivity meter
The diagram below illustrates a conductivity meter that can be used for testing conductivity of solutions or solids. It is possible for students to make the apparatus themselves. Alternatively students could be given the instruments ready-made.
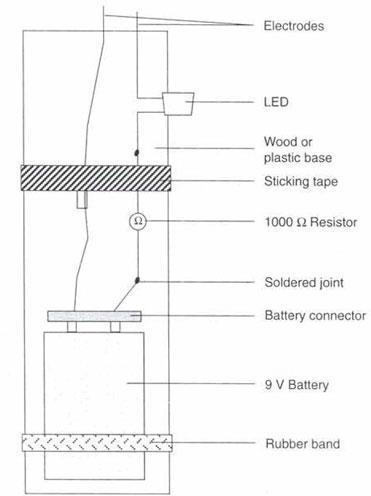
Apparatus
- Light-emitting diode (LED)
- Resistor, 1000 Ω
- Battery, 9 V
- Battery connector
- Piece of thin wood or plastic, approximately 150 × 25 mm
- Solder wire or tape
- Soldering iron
- Sticking tape
- Thick rubber band
2.5. Hoffman apparatus
Students can make their own microscale Hoffman apparatus from plastic pipettes, using it to investigate aspects of electrolysis.
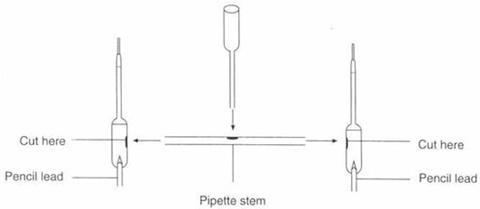
Apparatus
- Four plastic pipettes (standard form, eg Aldrich ref’ Z13,500-3)
- Scissors
- A pin
- Pencil leads (HB 0.9 mm)
- Adhesive, eg polystyrene glue
Procedure
- Using the scissors cut two holes in two of the pipettes as indicated in the diagram below.
- Cut off the stem from a third pipette and insert it in the first two pipettes to join them.
- Cut off the tip and end of the bulb of the fourth pipette and insert the tip end into a hole in the middle of the stem joining the first two pipettes.
- With the pin make a hole in the bottom of the two bulbs and carefully insert a pencil lead into each.
- Apply adhesive to each joint and leave to dry.
3. Preparing solutions
Download our solutions guide to find out how to prepare a variety of solutions for use in microscale experiments. The guide features compounds of a range of common elements, with instructions detailing the chemicals and quantities required, as well as hazard information relating to each solution.
Additional resources
CLEAPSS, SSERC and microchemuk also have a range of up-to-date resources and information relating to microscale chemistry.
Downloads
Guide to preparing solutions for microscale chemistry
Editable handout | Word, Size 52.38 kbGuide to preparing solutions for microscale chemistry
Handout | PDF, Size 0.15 mb
Additional information
This resource is part of our Microscale chemistry collection, which brings together smaller-scale experiments to engage your students and explore key chemical ideas. The resources originally appeared in the book Microscale chemistry: experiments in miniature, published by the Royal Society of Chemistry in 1998.
© Royal Society of Chemistry
Health and safety checked, 201


















1 Reader's comment