Make bubbles of carbon dioxide, hydrogen or methane in this demonstration exploring density, diffusion and solubility
In this experiment, students watch as bubbles of carbon dioxide – which sink in air – are created using dry ice. They then observe as hydrogen or methane is used to blow bubbles which float in air and can be ignited. Finally, mixtures of carbon dioxide and hydrogen can be used to blow bubbles which initially sink but eventually float.
This entertaining demonstration will suit open days, or lessons in which students discuss the properties of carbon dioxide or hydrogen. It touches on a number of key areas in chemistry, including density and solubility of gases, sublimation and combustion. Each of these areas could be explored in more detail, depending on the level of the group.
The time for carrying out the demonstration should be about ten minutes.
Equipment
Apparatus
- Eye protection for demonstrator
- Conical flask, 1 dm3
- Two-holed rubber stopper, to fit flask
- Plastic funnel, small – about 3 cm diameter
- Beaker, 100 cm3
- Expanded polystyrene container, such a cool box (for storing dry ice) (see note 7 below)
- Insulating gloves (for handling dry ice)
- Length of right-angled glass tubing (see figure 2)
- Length of plastic tubing or rubber tubing, about 50 cm
- Dropping pipette teat, to fit glass tubing
- Boss, clamp and retort stand, as required
Chemicals
- Candle or wax taper – taped to a stick about 1 m long
- Solid carbon dioxide (dry Ice), about 100 g (see note 7)
- Washing-up liquid, a few cm3
- Propane-1,2,3-triol (glycerol), a few cm3
- Source of hydrogen (EXTREMELY FLAMMABLE) and/or methane (Natural Gas) (EXTREMELY FLAMMABLE) (see note 8)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Solid carbon dioxide (dry ice), CO2 (s) – see CLEAPSS Hazcard HC020a.
- Propane-1,2,3-triol (glycerol), CH2 OHCH(OH)CH2 OH(l) – see CLEAPSS Hazcard HC037A.
- Hydrogen, H2 (g) (EXTREMELY FLAMMABLE) – see CLEAPSS Hazcard HC048.
- Methane, CH4 (g) (EXTREMELY FLAMMABLE) – see CLEAPSS Hazcard HC045a.
- Dry ice can be obtained from a local university, higher education institution or nearby industry. It can be stored and transported in an expanded polystyrene box and kept for several hours. The temperature of dry ice is approximately –80 °C. It can cause serious frostbite if wrongly handled. Use insulated, not rubber, gloves. It must never be stored in a sealed container and the container must not be kept in a confined space where evaporation might result in asphyxiation. Always ensure the container has a vent hole through which the gas can escape, to avoid the pressure building up. The ‘fluffy’ solid carbon dioxide that can be generated from a cylinder of the gas, using a special adaptor, is not suitable for this experiment. Alternatively carbon dioxide gas can be generated using a Kipp’s apparatus or the simple method described on in these standard techniques for generating, collecting and testing gases.
- A hydrogen cylinder fitted with a finely adjustable regulator should be used. Establish a suitable flow rate of hydrogen before connecting the supply to the bubble pipe. Alternatively a chemical generator could be used (see the guidance on generating, collecting and testing gases) but controlling the flow rate will be more difficult.
Procedure
Before the demonstration
- Make a bubble pipe by glueing a length of absorbent material, such as a loosely woven shoelace or a piece of thick wool, around the inner rim of a plastic funnel (see figure 1 below). This acts as a wick that soaks up and retains bubble mixture so that several bubbles can be blown without re-dipping the pipe into the bubble mixture.
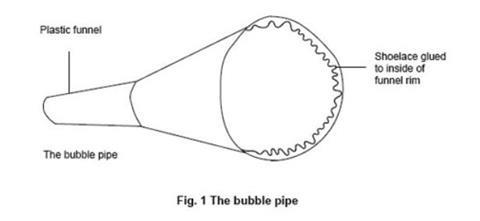
- Make about 50 cm3 of bubble mixture by mixing roughly 5 cm3 of washing-up liquid, 5 cm3 of glycerol and 40 cm3 of water. If another detergent, such as Teepol, is used, it is worth doing some preliminary experimentation to find a mixture that can produce robust bubbles with the gases used.
- Wind some insulating tape around the conical flask a precaution against explosion. Fit the flask with the stopper and tubes as shown in figure 2 below. Half fill the flask with water warmed to a few degrees above room temperature. Seal the inlet to the flask with a pipette teat. Clamping the flask in position near the edge of a bench will allow the heavier than air bubbles to sink to the floor when they detach.
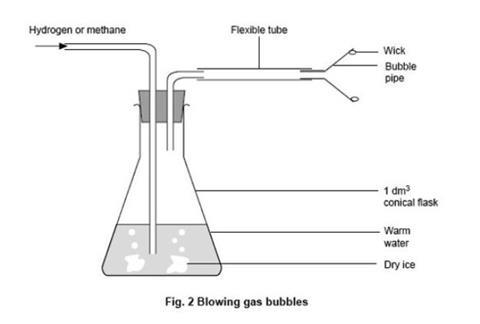
The demonstration
- Dip the bubble pipe into the bubble mixture so that the wick is soaked. Attach it to the flask outlet. Using gloves put a few small pieces of dry ice into the flask and re-stopper it. A ‘fog’ will form in the flask and fog-filled bubbles of carbon dioxide will be formed by the bubble pipe. The bubbles will sink rapidly to the bench top or the ground – a plastic tray on the floor will reduce the mess (and possible slipping hazard) and should allow some bubbles to settle without bursting. These bubbles will shrink on standing as carbon dioxide diffuses out of the bubble faster than the less soluble air diffuses in.
- When the dry ice is used up, remove the pipette teat and connect the hydrogen or natural gas supply to the flask. Turn on the gas supply carefully and blow more bubbles. The first few will sink, then they will start to float as the carbon dioxide is displaced from the flask. The floating bubbles can be ignited with a lighted candle on a stick, but take great care only to ignite bubbles well away from the bubble pipe and the flask, and any flammable material on the ceiling, including some types of ceiling tile.
- Turn off the gas supply and add more dry ice to the flask, to blow bubbles with a mixture of carbon dioxide and hydrogen. These will sink or float depending on the proportions of the two gases in the bubbles. With practice and some patience it is possible to blow bubbles that start to sink then, as the carbon dioxide diffuses out of them, float upwards. The best method is to start by blowing pure carbon dioxide bubbles, then gradually increasing the proportion of hydrogen until the bubble only just pulls downwards while still attached to the bubble pipe. The bubble can then be detached by shaking it gently from the pipe.
Teaching notes
The demonstration first of all shows the difference in density, relative to air, of the two gases.
The rate at which gases diffuse depends on their molecular masses (quantified in Graham’s Law of Diffusion). Thus hydrogen and methane diffuse faster than carbon dioxide or air. Students may have noticed that cheap, ordinary balloons filled with helium usually deflate much more quickly than the more expensive foil-lined type sold in various shapes, such as hearts. This is because helium diffuses through the cheap balloon’s rubber membrane more quickly than the air diffuses in. The material of more expensive balloons is less permeable to gases.
However, here the carbon dioxide bubbles shrink rather than expanding, the opposite of what would be expected based on rates of diffusion. Consider a sealed porous pot filled with carbon dioxide. The pressure in the pot would rise as the less dense air diffuses into the pot faster than the more dense carbon dioxide diffuses out. However, here diffusion is through the bubble membrane, consisting mostly of water. The greater solubility of carbon dioxide in water, compared to that of air, enables the carbon dioxide to pass out of the bubbles faster than the air can diffuse in. This results in the bubble shrinking.
As hydrogen and methane having similar solubilities in water to the gases in air, one might expect lighter than air bubbles to shrink in air as the lighter gas diffuses out faster than air diffuses in. The floating bubbles unfortunately seldom last long enough for this to be seen.
The sublimation of dry ice is worth pointing out here. A small lump left on the desk will disappear without melting, although it may become covered in some ice. Students may be aware of the use of dry ice to generate a fog at rock concerts, although this has largely been replaced by ‘smoke machines’ generating a fog of oil droplets. The fog generated by dry ice is not carbon dioxide gas, but droplets of water condensed from the air cooled by the dry ice (at approximately –80 °C).
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet