Encourage students to make and test predictions about the pattern of solubility among lead halides in this practical.
In this experiment, students make predictions about the solubility of different lead halides and what might happen when solutions containing lead ions and various halide ions are mixed. They then test their predictions using dilute solutions containing the relevant ions.
Students follow this up by testing the solubility of the lead halides when heated, and confirm the pattern by allowing the solutions to cool again.
This is a straightforward class practical, illustrating some of the trends within the compounds of Group 17 elements (the halogens). It is ideal for discussing and using ionic equations, as well as making predictions, explaining observations and understanding the solubility patterns amoung common ionic compounds.
The known patterns of solubilities and properties of Group 17 compounds will need to be familiar to students – if not these will need to be discussed first.
The practical itself will take about 25 minutes to the point at which the hot solutions are ready to cool down, plus however long it then takes for the precipitates to re-form on cooling.
Equipment
Apparatus
- Eye protection
- Boiling tubes (large test tubes), x3
- Test tube rack
- Test tube holder
- Beaker, 250 cm3
- Bunsen burner
- Heat resistant mat
Chemicals
The following solutions should be available in dropper bottles (see notes 7 and 8 below):
- Lead nitrate, 0.2 M (TOXIC, DANGEROUS FOR THE ENVIRONMENT), 20 cm3
- Potassium chloride, 0.2 M, 10 cm3
- Potassium bromide, 0.2 M, 10 cm3
- Potassium iodide, 0.2 M, 10 cm3
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout. Wash hands after using lead nitrate solution.
- Lead nitrate (TOXIC, DANGEROUS FOR THE ENVIRONMENT both as solid and as 0.2 M solution) – see CLEAPSS Hazcard HC057a and CLEAPSS Recipe Book RB053.
- Potassium chloride – see CLEAPSS Hazcard HC047b and CLEAPSS Recipe Book RB068.
- Potassium bromide – see CLEAPSS Hazcard HC047b and CLEAPSS Recipe Book RB068.
- Potassium iodide – see CLEAPSS Hazcard HC047b and CLEAPSS Recipe Book RB072.
- If dropper bottles are not available for dispensing solutions, students will also require dropping pipettes to dispense solutions.
- Sodium halides can be used instead of potassium halides if preferred.
Procedure
- Place three boiling tubes in a row in a test tube rack.
- To each tube add about a 3 cm depth of 0.2 M lead nitrate solution.
- To the first tube add 5 drops of 0.2 M potassium chloride solution, and note what happens. Keep the mixture formed for step 7 below.
- To the second tube add 5 drops of 0.2 M potassium bromide solution, and note what happens. Keep the mixture formed for step 7 below.
- To the third tube add 5 drops of 0.2 M potassium iodide solution, and note what happens. Keep the mixture formed for step 7 below.
- Heat each of the test tubes from steps 2, 3 and 4 in turn in a low Bunsen flame until the mixtures are boiling. Allow them to boil very gently for a minute.
- Allow the three boiling tubes to cool down. If time is short, they may be cooled by standing in a beaker of cold water.
- Observe and record what happens in each of the three tubes as the mixtures in them cool.
Teaching notes
This practical can be carried out either as an investigative experiment, as described above, or as a simple exploration without use of prior knowledge. In the latter case, students will not need to be familiar with solubility patterns of ions in solution and their reactions, or with ionic equations.
However, to make it an investigation, the points below need to be considered:
- Students will need some generalizations about the solubilities of salts before they can be asked to predict whether or not a precipitate will form on mixing two solutions and what the precipitate will be. If these have not been taught previously, the lesson may need to start with a minimum of these generalizations:
- All nitrates are soluble.
- All sodium and potassium salts are soluble.
- All chlorides are soluble except for the chlorides of lead and silver.
- All lead salts are insoluble in cold water except for the nitrate (and ethanoate).
- Students also need to be able to think in terms of cations and anions ions behaving independently in solution. Diagrams such as those below can be helpful.
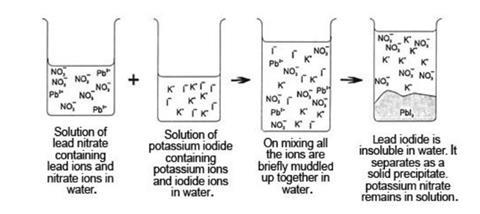
- Students need to be able to translate these diagrams into the formalities of chemical equations; eg the ionic equation:
Pb2+(aq) + 2I–(aq) →PbI2(s)
and the full equation:
Pb(NO3)2(aq) + 2KI(aq) →PbI2(s) + 2KNO3(aq)
The solubilities of the lead halides increase markedly with temperature, so that the three halides under investigation are all effectively soluble in boiling water. This means that on cooling these solutions, the lead halides will crystallise out again. For lead chloride and lead bromide, the effect is rapid and the crystals small, so their appearance returns to that of a precipitate.
However for lead iodide, especially on slower cooling, the effect of recrystallisation can be spectacular, with thin golden flaky crystals of lead iodide shimmering in suspension, and falling as golden rain to the bottom of the tube. If the students do not observe this phenomenon, it is well worth the teacher repeating this stage as a demonstration.
Related resources
Try a related experiment to illustrate the formation of silver and lead halides in a series of precipitation reactions.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet