This open-ended activity is perfect for learners to devise their own approaches to problem-solving in chemistry.
This experiment should take one to two hours.
Equipment
General
- A range of laboratory glassware including measuring cylinders, burettes and beakers
- Access to two decimal place balance
- Items from the junk list
- Eye protection
Materials per group
- Bottle of vinegar
- Magnesium ribbon cut into 0.04g pieces.
Health, safety and technical notes
Read our standard health and safety guidance here.
Wear eye protection.
This is an open-ended problem-solving activity, so the guidance given here is necessarily incomplete.
Commentary
There are many approaches to this problem, but they all share a common aim to measure the volume of hydrogen evolved from a known mass of magnesium. Most approaches collect the gas over water in a graduated device such as a measuring cylinder or burette.
Students need to consider the accuracy of the method that they choose. The volume of gas is noted and adjusted for atmospheric pressure. It is then simple to calculate the volume of 1 mole of the gas.
Mg(s) + 2CH3 COOH(aq) → (CH3 COO)2 Mg(aq) + H2 (g)
1 mole 1 mole
Evaluation of solution
This problem has been used as a competition. The best solution is the one that is nearest the theoretical value, but credit should also be given for elegance.
During trialling, many students forgot to adjust the volume of gas collected to atmospheric pressure and room temperature.
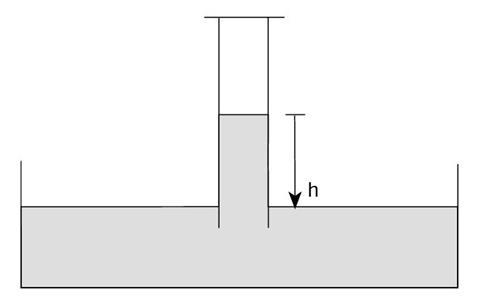
Pressure of gas + ρgh + SVP = atmospheric pressure
SVP = Saturated vapour pressure of water
ρ = density of water
h = height of water
The corrections are very small. The difference between theoretical and experimental answers is usually very small.
Notes
This resource is part of a collection of problem-solving activities, designed to engage learners in small group work. Find out how to use these resources, and obtain a list of suggested ‘junk items’ here.
Downloads
Gas volume
Experiment | PDF, Size 43.04 kb
Additional information
The resources were originally published in the book In Search of More Solutions.


















No comments yet