All Reactions and synthesis articles – Page 21
-
 Lesson plan
Lesson planReactions of acids with metals and carbonates | 11-14 years
Help your students unravel misconceptions about how acids react with metals and carbonates via this lesson plan with downloadable activities for ages 11–14.
-
 Lesson plan
Lesson planExploring precipitation reactions using diagrams | 14-16 years
Discover how ions are arranged in precipitation reactions and practise completing ionic equations using this lesson plan with activities for 14–16 year olds.
-
 Lesson plan
Lesson planHow does burning magnesium affect its mass? | 11-14 years
Investigate what happens to the mass of magnesium when it burns and reacts with oxygen using this lesson plan and practical activity for 11–14 year olds.
-
 Lesson plan
Lesson planHow do alkali metals react with water? | 14-16 years
Explore how alkali metals react with water using a series of demonstrations and videos in this lesson plan with activities
-
 Lesson plan
Lesson planWhat are chemical reactions used for? | 11-14 years
Try this lesson plan for 11–14 year olds to explore how chemical reactions are used to produce energy, make new materials or support biological systems.
-
 Lesson plan
Lesson planWhy do chemical reactions happen? | 16-18 years
Introduce students to entropy and explore why chemical reactions happen using role play, discussion and demonstrations in this lesson plan for 16–18 year olds.
-
 Lesson plan
Lesson planHow are particles rearranged when iron burns in air? | 11-14 years
Demonstrate the combustion of iron and explore how particles are rearranged to form iron oxide using this lesson plan with activities for 11–14 year olds.
-
 Lesson plan
Lesson planWhat happens to particles in chemical reactions? | 11-14 years
Explore what happens to atoms and molecules when new materials are made in chemical reactions, using this lesson plan with activities for 11–14 year olds.
-
 Lesson plan
Lesson planWhat is a chemical reaction? | 11-14 years
Explore the key idea that chemical reactions produce one or more new substances while conserving matter in this lesson plan with activities for 11–14 year olds.
-

-
 Exhibition chemistry
Exhibition chemistryExplosive nitrated carbon compounds
Demonstrations designed to capture the student's imagination
-
 Opinion
OpinionConcrete stalactites
Peter Borrows takes us on another excursion into local chemistry. In this issue: concrete stalactites
-
 Feature
FeatureWho really discovered the Haber process?
Although Fritz Haber's name is now attached to the process for the synthesis of ammonia from its constituent elements by using high pressure, who was responsible for this reaction?
-
 Exhibition chemistry
Exhibition chemistryDecomposing hydrogen peroxide with blood
Mixing hydrogen peroxide with blood to produce a foam explosive
-

-
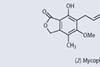 Feature
FeaturePhenols in medicine
Phenol encountered in school or college chemistry laboratories demands special respect on account of its toxic and corrosive nature. But phenol and its derivatives do have a few medicinal surprises
-

-
 Exhibition chemistry
Exhibition chemistryA spectacular reversible reaction
A demonstration with a dramatic colour change
-

-
 Feature
FeaturePain relief: from coal tar to paracetamol
Analgesics, ie pain-relieving drugs, fall into two categories: those that also reduce body temperature in fevers (antipyretics), and those that act mainly on the brain - typically morphine and diamorphine/heroin. Here we consider members of the first group, particularly those once designated 'coal tar analgesics'. Paracetamol, our most popular over-the-counter pain killer, is one of these.











