Make Bakelite in the classroom and investigate its properties using phenol, methanal and ethanoic acid in this demonstration
In this experiment, students observe as phenol is dissolved in a mixture of aqueous methanal and ethanoic acid in a throwaway container. Acidification of this solution initiates condensation polymerisation, and a pink thermosetting polymer is formed within a few minutes. This is Bakelite, the first genuine synthetic polymer. After washing the product, the demonstrator can investigate its properties, illustrating these to the class.
If a mould is used, the experiment can be extended to show the formation of plastic articles made in a mould by condensation polymerisation.
This is a teacher demonstration, taking about five minutes for one polymerisation experiment. Extensions to show production of moulded articles will take longer. It should be performed in a fume cupboard to avoid exposure to pungent vapours from the reaction mixture.
Equipment
Apparatus
- Eye protection (goggles)
- Disposable nitrile gloves (for handling the polymer)
- Access to a fume cupboard visible to the class
- Measuring cylinder, 100 cm3
- Beaker, 100 cm3, 250 cm3, 1 of each
- Throwaway containers with secure lids, at least x2 (see note 7 below)
- Stirring rod
- Spatula
- Plasticine
- Tongs
- Object of simple shape for making suitable mould (see note 8)
- Bunsen burner (optional)
Chemicals
- Methanal solution (formalin), 37–40%, (TOXIC), 25 cm3
- Phenol (TOXIC, CORROSIVE), 20 g (see note 9)
- Ethanoic acid, pure (glacial acetic acid) (CORROSIVE), 55 cm3
- Sulfuric acid, concentrated (CORROSIVE), 30 cm3 (see note 10)
- Aluminium foil
Health, safety and technical notes
- Read our standard health and safety guidance.
- Work in a fume cupboard, and wear eye protection (goggles) and disposable nitrile gloves throughout.
- Methanal solution, HCHO(aq), (TOXIC) – see CLEAPSS Hazcard HC063. Methanal solution (also known as formaldehyde solution, or formalin) should be in good condition. Because the vapour is unpleasant, the supply of this solution for this demonstration should be kept in the fume cupboard, in a stoppered bottle.
- Phenol, C6H5OH(s), (TOXIC, CORROSIVE) – see CLEAPSS Hazcard HC070A.
- Ethanoic acid (glacial acetic acid), CH3COOH(l), (CORROSIVE) – see CLEAPSS Hazcard HC038a.
- Concentrated sulfuric acid, H2SO4(l), (CORROSIVE) – see CLEAPSS Hazcard HC098a.
- The container used should be transparent, disposable, and preferably should have a secure lid. Used 100 g coffee jars with screw tops are ideal, but any similar size glass jar will do.Disposal: after the demonstration, dispose of the screw top container with the polymer inside as solid waste.
- A mould for casting copies of an object can be made by pressing the object into plasticine. The object selected should have a simple external shape. Enough plasticine should be provided to press the shape into, forming a sufficiently substantial mould to retain its shape when handled.
- Before the lesson, weigh out 20 g of phenol into small beaker, from which it may be easily tipped into the disposable container.
- Before the lesson, add 30 cm3 of concentrated sulfuric acid to 30 cm3 of water slowly in a beaker, with constant stirring. Cool the resulting diluted acid to room temperature.
Procedure
- Pour 25 cm3 of aqueous methanal (formalin) into the disposable container and add 55 cm3 of glacial ethanoic acid.
- Add 20 g of phenol to the solution and stir to dissolve.
- Add 60 cm3 of the diluted sulfuric acid to the mixture with continuous stirring. The mixture will turn pale yellow, then suddenly pink and opaque.
- Stir steadily. Within a minute or so a pink solid will gather around the stirring rod. A lot of heat is evolved.
- Pour off the remaining milky liquid and rinse the pink polymer several times with water.
- Show that the polymer is hard by poking the material with a spatula.
- Before passing the material round the class, place the screw top securely on the jar as the product will be contaminated by unreacted starting materials. It may be necessary to break off the stirring rod.
- Make a plasticine mould from the simple shape and line it with aluminium foil.
- Make another phenol-methanal solution as above, but, immediately after adding the sulfuric acid, pour some of the solution into the mould and allow it to polymerise.
- Hold a sample of the washed polymer with tongs and heat in a Bunsen flame. It will char but will not melt showing that it is thermosetting.
Teaching notes
For an alternative procedure, see CLEAPSS Hazcards HC063 and PX000 (63 – Methanal).
The polymer produced is known commercially as Bakelite, the first genuine synthetic polymer (as opposed to modified natural polymers such as Celluloid, or modified cellulose). It was developed by Leo Bækeland in the early 1900’s, and it is still used to make articles such as electrical fittings.
The reaction is a condensation polymerisation in which water is eliminated as a hydrogen atom from the benzene ring of each of two neighbouring phenol molecules combines with the oxygen atom from a methanal molecule. The remaining –CH2– group from the methanal molecule then forms a bridge between two neighbouring phenol molecules. This process, repeated many thousands of times, forms chains of phenol and methanal molecules linked in this way.
Sometimes a second hydrogen atom from the ring of a phenol molecule will also react with a methanal molecule, producing a branch in the chain, and chains may become cross-linked to each other in the same way. Notice that the hydrogen atom of the phenolic O-H group is not directly involved, although it exerts a strong influence on the course of the reaction. Eventually a random three-dimensional network of cross-linked chains is formed, giving a rigid structure and thus a hard, inflexible material.
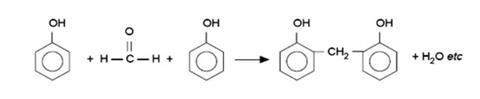
The product has considerable crosslinks, including some −CH2OCH2− linkages:
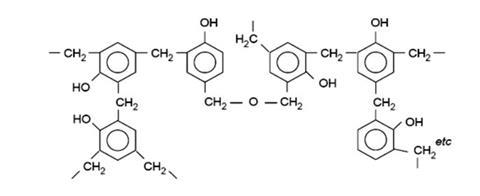
Because the tangle of cross-linked chains is almost impossible to separate, the material does not melt on heating although it will eventually break down at high temperature, giving off small molecules such as steam, and leaving a charred mass which is largely carbon.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet