Some post-16 students were unable to explain what was happening at the level of particles when precipitation occurs
Findings suggest students may not understand what happens during dissolving, and often have alternative conceptions of the nature of ionic bonding.
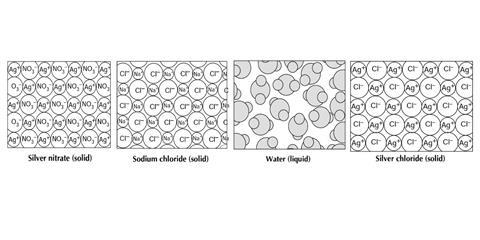
A reaction to form silver chloride
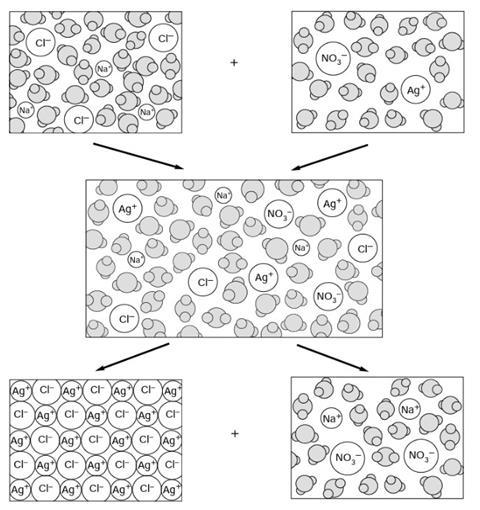
Silver chloride is an ionic solid. It can be prepared by reacting silver nitrate solution and sodium chloride solution. The diagrams represent the types of particles present in some of the substances involved in the reaction.
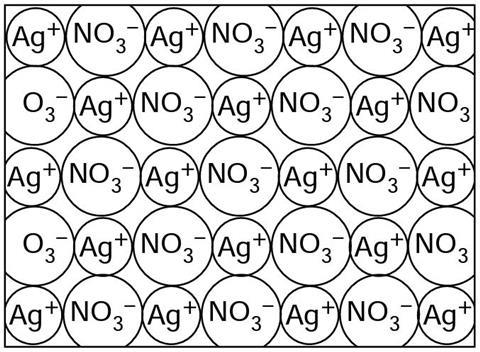
The diagram shows the particles in solid silver nitrate. The particles in silver nitrate are silver ions (Ag+) and nitrate ions (NO3–).
The diagram shows the particles in solid sodium chloride. The particles in sodium chloride are sodium ions (Na+) and chloride ions (Cl–).
The diagram shows the particles in water. Water is a liquid. The particles are water molecules.
The diagram shows the particles in solid silver chloride. The particles in silver chloride are silver ions (Ag+) and chloride ions (Cl–).
Questions
- Sodium chloride dissolves in water to give sodium chloride solution. What particles (such as particular atoms, molecules, ions) do you think are present in sodium chloride solution?
- Silver nitrate dissolves in water to give silver nitrate solution. What particles (such as particular atoms, molecules, ions) do you think are present in silver nitrate solution?
- When sodium chloride solution is mixed with silver nitrate solution a white solid forms. The following reaction take place: sodium chloride(aq) + silver nitrate(aq) →sodium nitrate(aq) + silver chloride(s) The solid that is formed is silver chloride. The solid can be separated from the liquid by filtration. The liquid that is left after filtration contains sodium nitrate. What particles (such as particular atoms, molecules, ions) do you think are present in the liquid after it is filtered?
- What do you think happens to the particles in the mixture when the ionic bonds form in the silver chloride in this reaction?
- How many chloride ions do you think are bonded to each silver ion in silver chloride? (Give the reason for your answer, if you can.)
A precipitation reaction
This exercise is about what happens during a precipitation reaction. When solutions of sodium chloride and silver nitrate are mixed, then a white solid (silver chloride) forms: sodium chloride(aq) + silver nitrate(aq) →sodium nitrate(aq) + silver chloride(s) Fill in the gaps below. Use the diagrams to help you.
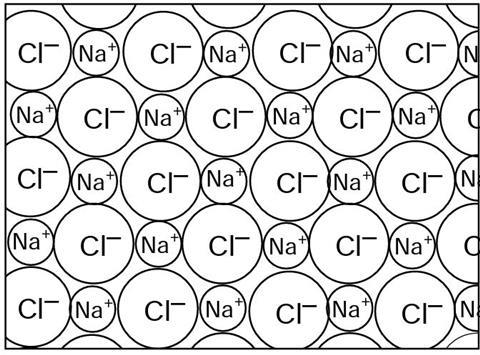
Sodium c_____________ is an ionic solid. Sodium ions (Na+) and chloride ions (Cl–) are bonded together by the e______________ attraction between the positive and negative ions. Each i______________ is attracted to each of those counter ions surrounding it. This type of chemical b___________ is called ionic bonding.
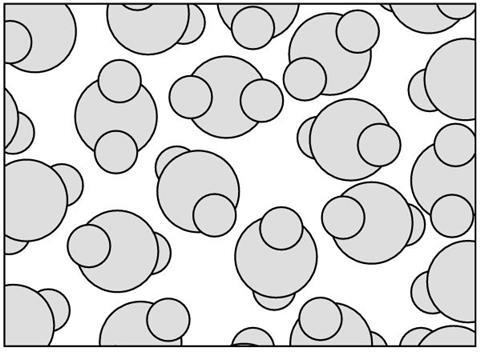
Sodium chloride dissolves in water. W___________ is a liquid containing water molecules. The m___________ move around quickly, bumping into each other. In these c___________ the water molecules bounce off one another. Many ionic solids will d___________ in water.
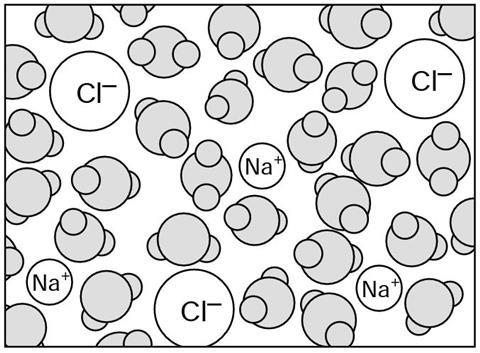
When the sodium chloride dissolves it forms a s__________. The solution contains the water molecules, and the s__________ ions and the c__________ ions from the sodium chloride. The fast m___________ water molecules constantly collide with the ions, and crowd around (‘solvate’) them, so that the i__________ can not stick together.
Silver nitrate is an i__________ solid. Silver ions (Ag+) and n__________ ions (NO3–) are bonded together by the electrical a__________ between the p__________ and negative ions. Each ion is attracted to each of those surrounding it. This type of c__________ bonding is called ionic bonding.
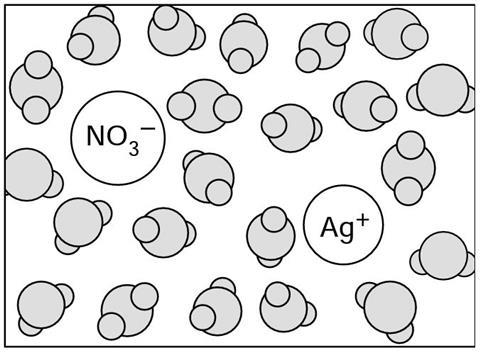
When the silver nitrate d__________ it forms a solution. The solution contains the water m__________, and the silver ions and the nitrate i_________ from the silver nitrate. The f__________ moving water molecules constantly c_______ with the ions, and crowd around (‘s__________’) them, so that the ions can not stick together.
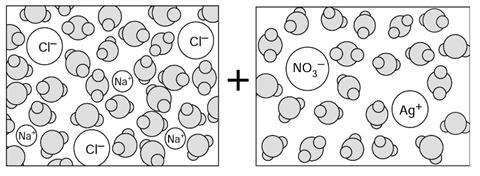
What do you think would be in the mixture if the two solutions below were to be poured into the same beaker?
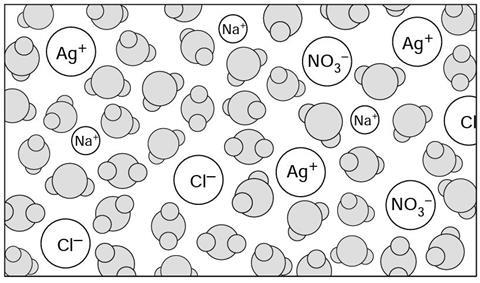
When the two solutions are m____________ together the new mixture contains water m____________, sodium ions, s____________ ions, chloride ions and nitrate i__________.
When silver ions collide with c____________ ions they sometimes stick together. The a_____________ between these ions is so strong that collisions with the fast moving w_____________ molecules do not stop them bonding together.
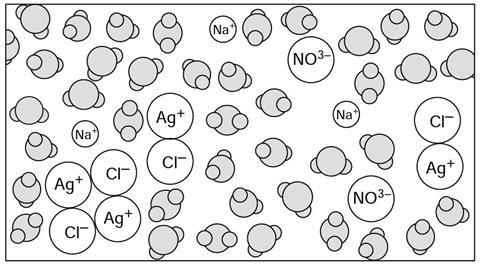
The silver ions and c______________ ions soon form into large enough crystals to p_______________ out from the solution. The solid precipitate of silver chloride sinks to the b_______________ of the mixture.
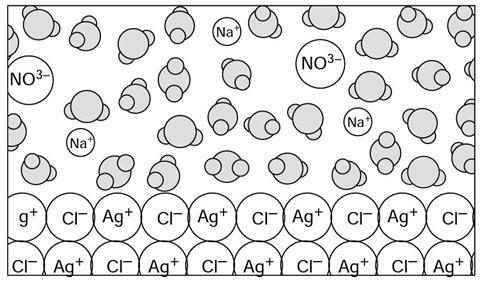
In the p__________ silver ions (Ag+) and chloride ions (Cl–) are b___________ together by the electrical attraction between the positive and negative i___________. Each ion is attracted to e_____________ of those surrounding it. Silver chloride is an example of a compound with ionic bonding which does not dissolve in water (it is insoluble in w_____________).

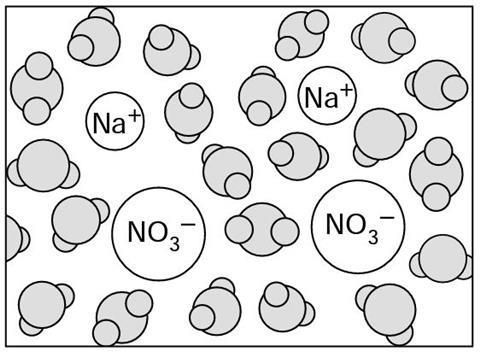
The liquid above the precipitate contains the water m___________, sodium ions and nitrate ions. This is a solution of sodium n___________. The fast moving water molecules constantly collide with the ions, and crowd around (‘solvate’) them, so that the ions can not s____________ together.
The real change during the reaction is that s_____________ silver ions and solvated c____________ ions form solid s____________ chloride:
Cl–(aq) + Ag+(aq) →AgCl(s)
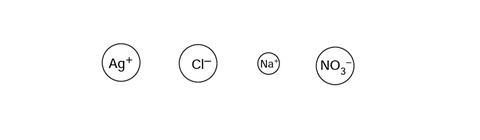
The sodium ions and nitrate ions are sometimes called ‘spectator’ ions because they are not directly involved in forming the product. Label the spectator ions in the box below:
Summary
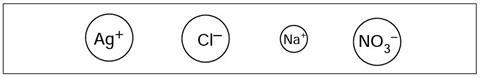
The change that has taken place in this reaction is more obvious if you use a colour key for the ions.
Select four different colours (eg blue, green, red and yellow), and colour each different type of ion in a different colour.
Use the box below as a key.
A reaction to form lead iodide
Lead iodide is an ionic solid. It can be prepared by reacting lead nitrate solution and potassium iodide solution.
- Potassium iodide dissolves in water to give potassium iodide solution. What particles (such as particular atoms, molecules, ions) do you think are present in potassium iodide solution?
- Lead nitrate dissolves in water to give lead nitrate solution. What particles (such as particular atoms, molecules, ions) do you think are present in lead nitrate solution?
- When potassium iodide solution is mixed with lead nitrate solution a yellow solid forms. The following reaction take place: potassium iodide(aq) + lead nitrate(aq) →potassium nitrate(aq) + lead iodide(s) The solid that is formed is lead iodide. The solid can be separated from the liquid by filtration. The liquid that is left after filtration contains potassium nitrate. What particles (such as particular atoms, molecules, ions) do you think are present in the liquid after it is filtered?
- What do you think happens to the particles in the mixture, when the ionic bond forms in the lead iodide in this reaction?
- How many iodide ions do you think might be bonded to each lead ion in lead iodide? (Give the reason for your answer, if you can.)
Answers
A reaction to form silver chloride
- H2O molecules, Na+, Cl–ions (also allow H+or H3O+and OH–ions, as long as H2O molecules given).
- H2O molecules, Ag+, NO3–ions (also allow H+or H3O+and OH–ions, as long as H2O molecules given).
- H2O molecules, Na+, NO3–ions (also allow H+or H3O+and OH–ions, as long as H2O molecules given). NB unless care is taken with reacting quantities the final solution may also contain either silver ions or chloride ions - but not both.
- The important point is that the silver ions and chloride ions are already present in the mixture, and the reaction involves the electrical attraction causing the ions to clump together and form crystals. (Answers about ion formation through electron transfer are wrong.)
- Students (especially those in the 14–16 age range) will not be expected to know about the precise crystal structure of silver chloride. Answers which suggest that the number of bonded ions depends upon the number of neighbours should be considered correct. For example, students may infer from the diagram that each ion is bonded to 4 or 6 others. Answers that are based on the charges on the ions (ie each silver ion is bonded to one chloride ion) are wrong, and may be related to irrelevant and inappropriate arguments about electron transfer.
A precipitation reaction
In this DART type activity students have to complete missing words from the initial letters. The ‘missing’ words are shown here as bold text:
Sodium chloride is an ionic solid. Sodium ions (Na+) and chloride ions (Cl–) are bonded together by the electrical attraction between the positive and negative ions. Each ion is attracted to each of those counter ions surrounding it. This type of chemical bonding is called ionic bonding.
Sodium chloride dissolves in water. Water is a liquid containing water molecules. The molecules move around quickly, bumping into each other. In these collisions the water molecules bounce off one another. Many ionic solids will dissolve in water.
When the sodium chloride dissolves it forms a solution. The solution contains the water molecules, and the sodium ions and the chloride ions from the sodium chloride. The fast moving water molecules constantly collide with the ions, and crowd around (‘solvate’) them, so that the ions can not stick together.
Silver nitrate is an ionic solid. Silver ions (Ag+) and nitrate ions (NO3–) are bonded together by the electrical attraction between the positive and negative ions. Each ion is attracted to each of those surrounding it. This type of chemical bonding is called ionic bonding.
When the silver nitrate dissolves it forms a solution. The solution contains the water molecules, and the silver ions and the nitrate ions from the silver nitrate. The fast moving water molecules constantly collide with the ions, and crowd around (‘solvate’) them, so that the ions can not stick together.
In the mixture there would be:
water molecules, sodium cations, chloride anions, silver cations and nitrate anions
When the two solutions are mixed together the new mixture contains water molecules, sodium ions, silver ions, chloride ions and nitrate ions.
When silver ions collide with chloride ions they sometimes stick together. The attraction between these ions is so strong that collisions with the fast moving water molecules do not stop them bonding together.
The silver ions and chloride ions soon form into large enough crystals to precipitate out from the solution. The solid precipitate of silver chloride sinks to the bottom of the mixture.
In the precipitate silver ions (Ag+) and chloride ions (Cl–) are bonded together by the electrical attraction between the positive and negative ions. Each ion is attracted to each of those surrounding it. Silver chloride is an example of a compound with ionic bonding which does not dissolve in water (it is insoluble in water).
The liquid above the precipitate contains the water molecules, sodium ions and nitrate ions. This is a solution of sodium nitrate. The fast moving water molecules constantly collide with the ions, and crowd around (‘solvate’) them, so that the ions can not stick together.
The real change during the reaction is that solvated silver ions and solvated chloride ions form solid silver chloride:
The sodium ion and the nitrate ion (in the box at the bottom of the page) should be labelled as ‘spectator ions’.
Summary sheet
Four different colours should be used to shade the four ions in the key (at foot of page). The ions in the other 5 figures should be coloured using the same colour code to show that the ions have effectively ‘swapped partners’.
It may be useful to make an overhead transparency of this page, and colour the ions. In this case the teacher may wish to set the colour code for the different ions – eg Na+blue, Ag+green, Cl–yellow, NO3–red – so that each student can readily check their summary sheet against the version displayed on the overhead projector.
A reaction to form lead iodide
- H2O molecules, K+, I–ions (also allow H+or H3O+ions and OH–ions, as long as H2O molecule is given).
- H2O molecules, Pb2+, NO3– ions (also allow H+or H3O+ions and OH–ions, as long as H2O molecule is given).
- H2O molecules, K+, NO3–ions (also allow H+or H3O+ions and OH–ions, as long as H2O molecule is given).
- The lead ions and nitrate ions are already present in the mixture, and the reaction involves the electrical attraction causing the ions to clump together and form crystals.
- Any answer that suggests that the number of bonded ions depends on the number of neighbours should be marked correct. Answers that are based on the charges of the ions are wrong.
Notes
For the full version of this chapter, see downloads below.
Downloads
Precipitation
PDF, Size 1.27 mb
Websites
Additional information
These resources have been taken from the book, Chemical Misconceptions : Prevention, diagnosis and care: Theoretical background, Volume 2, by Keith Taber.

Chemical misconceptions

Discover classroom strategies and activities to tackle common misconceptions among students in chemistry, and explore the theory behind different approaches.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
 Currently
reading
Currently
reading
Precipitation
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36





























































































No comments yet