Reaction mechanisms require students to appreciate the electrical changes which are conjectured to accompany chemical reactions
And also to learn a new formalism where the movement of electrons or electron pairs is represented by full or half arrows, which may start and end on either atoms or bonds.
Reaction mechanisms
Chemists use reaction mechanisms to show what they think might be happening as molecules interact during chemical reactions.
When drawing reaction mechanisms, the chemist usually assumes:
- The reaction occurs in several distinct steps;
- That each step can be represented as the movement of electrons; and
- That sometimes electrons move as pairs, and sometimes they move individually.
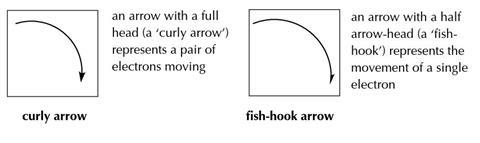
Diagrams showing the steps in reaction mechanisms usually show the molecules and/or ions (shown by + and –) and/or radicals (shown by •) involved, as well as arrows showing the movement of electrons. Two types of arrows are used:
There are two questions in this exercise. The questions each consist of a central diagram showing the initial stage in a reaction mechanism, surrounded by a selection of suggestions for the result of that step. Your task in each case is to identify which of the diagrams gives the correct outcome of that reaction step. Draw a large arrow showing which diagram is correct, as in the example below.
Try and explain your reason(s) for selecting the diagram you chose.
Reaction mechanism 1
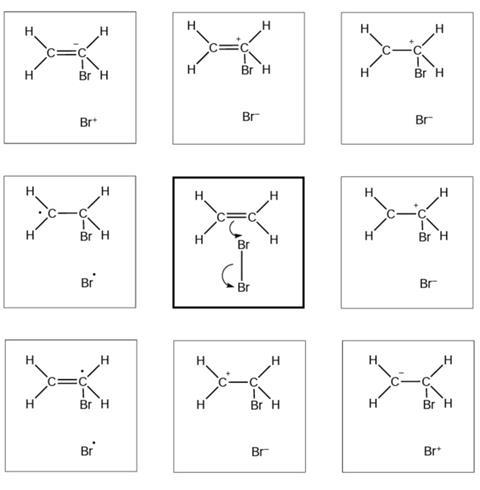
- I selected this diagram because …
Reaction mechanism 2
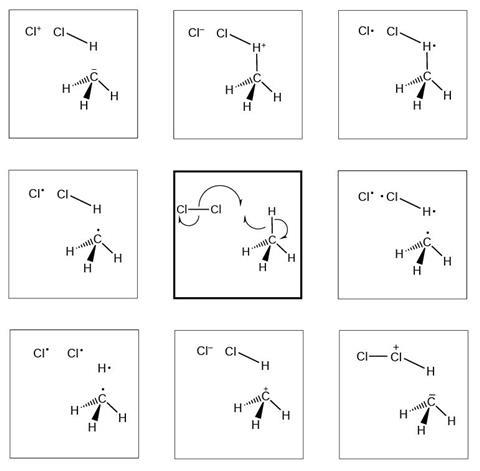
- I selected this diagram because…
Reaction mechanism revealed
Reaction mechanism 1
The diagrams below show and explain the correct answer to the question about the ionic reaction mechanism.
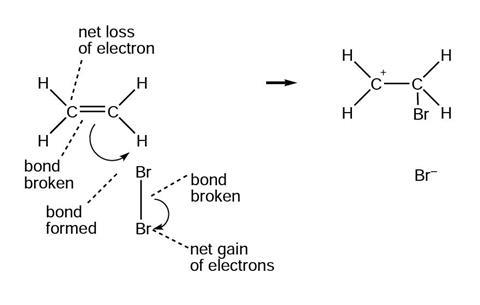
Reaction mechanism 2
The diagrams below show and explain the correct answer to the question about the free radical mechanism.
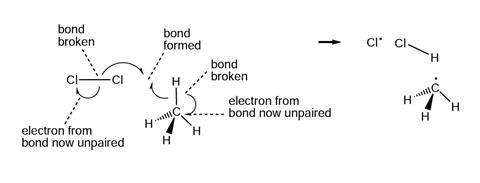
Notes
For the full version of this chapter, see downloads below.
Downloads
Reaction mechanisms
PDF, Size 0.4 mb
Websites
Additional information
These resources have been taken from the book, Chemical Misconceptions : Prevention, diagnosis and care: Theoretical background, Volume 2, by Keith Taber.

Chemical misconceptions

Discover classroom strategies and activities to tackle common misconceptions among students in chemistry, and explore the theory behind different approaches.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
 Currently
reading
Currently
reading
Reaction mechanisms
- 34
- 35
- 36





























































































No comments yet