Calcium carbonate is strongly heated until it undergoes thermal decomposition to form calcium oxide and carbon dioxide
This experiment can be carried out conveniently in groups of two or three and takes about 40–45 minutes.
Equipment
Apparatus
- Eye protection
- Tripod
- Gauze
- Bunsen burner
- Tongs
- Boiling tubes, x2 (note 1)
- Drinking straw (note 2)
- Dropping pipette
- Filter funnel, small
- Filter paper
Apparatus notes
- Use large (150 x 25 mm) test tubes (boiling tubes).
- Freshly purchased drinking straws should be used and each student issued with their own straw.
Chemicals
- Calcium carbonate
- Universal Indicator solution (HIGHLY FLAMMABLE)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection.
- Calcium carbonate, CaCO3(s) – see CLEAPSS Hazcard HC019b. The calcium carbonate used should be in the form of pea sized lumps of chalk. Blackboard chalk should not be used as it is likely to be mostly calcium sulfate.
- Universal indicator solution (HIGHLY FLAMMABLE) – see CLEAPSS Hazcard HC032 and CLEAPSS Recipe Book RB047.
Procedure
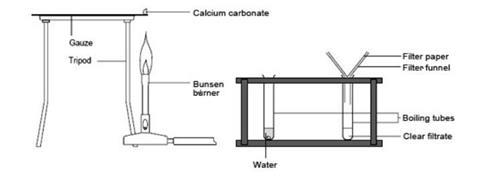
- You need to prepare a tabulated results sheet before you start your experiments. An example table is provided below in the teaching notes.
- Set a lump of chalk (calcium carbonate) on a gauze. If your gauze has a coated central circle, use the edge where there is no coating.
- Heat the chalk very strongly for 5–10 minutes. Write down what you observe.
- Let the chalk cool and use tongs to move it into a boiling tube. Add 2–3 drops of water with a dropping pipette. Write down your observations.
- Add about 10 cm3 more water to the solid. What happens now?
- Filter half the mixture into the other boiling tube and, using a straw, gently blow a stream of bubbles through the filtrate. What do you see?
- Test the remaining half of the mixture with Universal Indicator solution. Write down what you observe.
Teaching notes
Keep an eye on less mature students who might be tempted to suck rather than blow through the filtrate.
The results expected are as follows:
|
Method |
Observation |
|
Heat for 10 minutes |
The chalk should be seen to crumble slightly |
|
Add 2–3 drops of water |
More crumbling, steam given off, evidence that mixture has become hot |
|
Add 10 cm3 more water |
Some of the solid dissolves, white suspension |
|
Blow bubble through the solution |
Limewater turns cloudy |
|
Add universal indicator |
Indicator goes from green to blue/purple |
This set of experiments involves a variety of important reactions and types of reactions, with several references to industrial processes. The roasting of limestone and the hydration of the quicklime formed has relevance in the manufacture of plaster and cement, and in the laboratory limewater is a common reagent for the testing of carbon dioxide. Students could be asked to carry out web research on these applications.
Some question and answers for the class after the experiment
- Why does the chalk crumble slightly on strong heating?
Carbon dioxide/a gas is evolved; this forces its way out of the solid and breaks down its structure. - What type of reaction is taking place during the heating process? Write an equation for the reaction.
Thermal decomposition; CaCO3(s) → CaO(s) + CO2(g) - Why is steam evolved when drops of water are added? Write an equation for the reaction occurring.
The reaction is highly exothermic and the small amount of water added is partly converted to steam in the process: CaO(s) + H2O(l) → Ca(OH)2(s) - Why does the limewater turn cloudy? Write an equation for the reaction which is occurring.
Insoluble calcium carbonate is being precipitated: Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l) - What does the colour change occurring when limewater is added tell you about the pH of the solution? Explain why the pH would be expected to have this value.
The pH is about 11 - 14; soluble metal hydroxides are alkaline and therefore give high pH values.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet