Qiuz your students on trends in the periodic table
The topics covered in Starter for ten activity are: group 3 trends – melting points, ionisation energies and atomic radius; group 2 and group 7.
Example questions
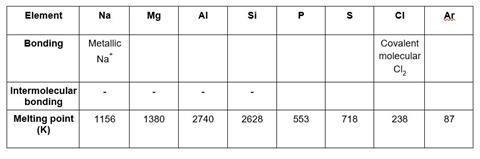
- Fill in the table above to show how melting point changes across Period 3 according to bonding type.
- Explain the differences in melting point between the following pairs of elements
(a) Magnesium and aluminium
(b) Phosphorus and sulfur
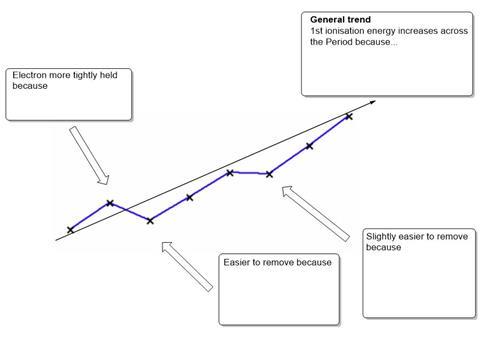
- The diagram shows the trend in 1st ionisation energy across the Period 3 elements. Complete the diagram giving the explanations for the trends seen.
- Define the term 1st ionisation enthalpy and illustrate with an equation.
Notes
The full resource, containing questions and answers, is available from the ‘Downloads’ section below. An editable version is also available.
Downloads
Trends in the periodic table - editable version
Word, Size 0.45 mbTrends in the periodic table
PDF, Size 0.42 mb
Starters for 10: Advanced level 1 (16–18)

This chapter in our Starters for ten series covers quantitative chemistry, atomic structure, bonding, trends in the periodic table, organic chemistry, thermodynamics, kinetics, equilibria, redox, analysis and experimental skills.
- 1
- 2
- 3
- 4
 Currently
reading
Currently
reading
Trends in the periodic table
- 6
- 7
- 8
- 9
- 10
- 11
- 12









































No comments yet