Add some zest to your lessons on this topic with these classroom tips, activities and ideas to combat misconceptions
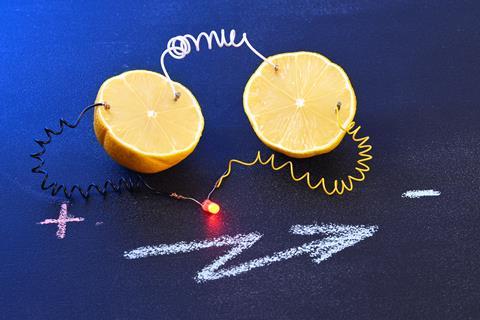
When teaching electrochemistry, I often refer to the adage: ‘when life gives you lemons, make lemonade’. I prefer to offer my students a more practical alternative: making a lemon clock.
When teaching electrochemistry, I often refer to the adage ‘when life gives you lemons …’, usually followed by ‘… make lemonade’. I prefer to offer my students a more practical alternative: making a lemon clock (bit.ly/3rjHpVR).
Explore the chemistry behind the process to help students relate their prior knowledge of the reactivity series, redox processes and writing half-equations to understand what’s happening at each electrode. This will help them get to grips with how simple electrochemical cells work.
Electrochemical cells have a wide variety of real-world applications, particularly their potential use as biosensors. They offer a highly sensitive, rapid alternative to conventional methods for testing for autoantibodies, which are crucial in providing a first defence against our own cells or tissues, with applications in cancer treatment.
Interacting with different representations of Johnstone’s triangle using modelling is a fantastic way to boost student understanding
When teaching this topic, it is crucial to be clear in your explanations, confident in your use of ionic and half-equations, and able to anticipate pitfalls. This challenging area has taken me years to teach with absolute confidence. Preparation is key. Brush up on your subject knowledge by first reviewing your exam board’s specification. There are nuances – each specification has a slightly different focus on what they want students to know, apply and understand, so start from a point of clarity.
Common misconceptions
Research shows that electrochemical cells and related topics are particularly challenging for pupils because they require the simultaneous use of several ideas. Underlying issues can easily propagate if left unchecked. It’s essential to both thoroughly prepare before each lesson and to take time to carefully review student work afterwards.
This topic has several pinchpoints, so ensure you plan in time to identify, diagnose and address these. I have found the RADAAR approach helpful. By scouring the examiner reports from recent A-level examinations, I identified some key pinchpoints and split them into three categories.
- The cells: how they’re set up; drawing labelled diagrams; function and use of salt bridges; their equilibriums
- Redox equations: writing and combining half-equations to show the feasible direction; identifying oxidising and reducing agents
- Calculation work: how to calculate Ecell; explaining feasibility using data; the electrochemical series
Download this
Electrochemical cells misconception buster, for age range 16–18
Quiz your learners’ knowledge with multiple choice questions and differentiated follow-up tasks, from the Education in Chemistry website: rsc.li/3rlbMew
Ideas for your classroom
To successfully teach this topic, it’s critical to plan in regular check-ins to probe students’ understanding. Below, I explore each of the three pinchpoints by employing a mini version of the RADAAR planning logic, with suggestions of how to diagnose and overcome these.
The cells
As with many aspects of chemistry, interacting with different representations of Johnstone’s triangle using modelling is a fantastic way to boost student understanding of: macroscopic observations when half-cells are connected; what’s happening at a sub-microscopic level in terms of redox; the symbolic equation work which can represent this. Critical to this is the interaction between practical observations and the use of modelling approaches to reinforce the chemistry behind the observations. You can use chemistry vignettes with visuals highlighting the three points of Johnstone’s triangle to boost understanding.
As with many aspects of chemistry, interacting with different representations of Johnstone’s triangle using modelling is a fantastic way to boost student understanding of: macroscopic observations when half-cells are connected; what’s happening at a sub-microscopic level in terms of redox; the symbolic equation work which can represent this. Critical to this is the interaction between practical observations and use of modelling approaches to reinforce the chemistry behind the observations. You can use chemistry vignettes (rsc.li/3CWzLDy) with visuals highlighting the three points of Johnstone’s triangle to boost understanding.
It is perhaps unsurprising that this topic also stretches us as teachers to our very limits

Start by reinforcing students’ prior knowledge of redox – I usually use the familiar example of the displacement reaction which occurs when copper wire is added to silver nitrate. From this, students should be able to construct half-equations, identify oxidation and reduction, and explain the idea of electron flow.
To move this learning forward, explore how to use this electron transfer in electrochemical cells. I model it with clear diagrams for the two half-cells, detailing how they are constructed, then making these electrochemical cells practically and measuring the voltage produced. You can monitor student understanding with multiple choice questions to diagnose areas of misconception within the cell. Common examples are the function of salt bridges, direction of electron flow – which cell goes on the left and which the right – and details of how the cell is set up using standard conditions and conventional representations.
To move this learning forward, explore how to use this electron transfer in electrochemical cells. I model it with clear diagrams for the two half-cells, detailing how they are constructed, then making these electrochemical cells practically (rsc.li/3rfIMoL) and measuring the voltage produced. You can monitor student understanding with multiple choice questions to diagnose areas of misconception within the cell. Common examples are the function of salt bridges, direction of electron flow – which cell goes on the left and which the right – and details of how the cell is set up using standard conditions and conventional representations.
Redox equations
Research studies identify student misconceptions in many areas of redox equations: loss and gain of electrons; increase and decrease in oxidation number; identifying oxidation and reduction; identifying oxidising and reducing agents. So it’s crucial that you continually model it, checking and reviewing throughout lessons.
I use quick, five-minute diagnostic tests at the beginning of lessons, giving students an equation, a cell diagram or the electrochemical series and asking them to identify a variety of these concepts. These tests highlight student misconceptions, allowing me to flex my lesson accordingly and revisit the necessary ideas. Using multimodal communication across Johnstone’s triangle reinforces the concepts thoroughly. My students may begin to roll their eyes when they see this task used time and time again. That shows me it’s working.
Calculation work
I model all calculation work visually using number grids to represent the reactions occurring. This allows students to map the sub-microscopic ideas of electron flow to the symbolic use of equations, allowing them to understand the chemistry behind their macroscopic observations. I have found the use of visuals invaluable in teaching this topic, and getting students to draw what they think is happening can be very useful in identifying misconceptions.
Checking for understanding
You can use diagnostic questions, handily broken down into key misconceptions, to effectively probe student understanding throughout the topic. And you can also reinforce their knowledge by giving them a variety of redox activities to complete.
Checking for understanding
- Probe student understanding throughout the topic with these diagnostic questions: rsc.li/3rlbMew
- Reinforce student knowledge with a variety of redox activities: rsc.li/44s8N2k
Take-home points
Electrochemistry is an area of the specification designed to stretch students, so it’s perhaps unsurprising that this topic also stretches us as teachers to our very limits. Paramount to teaching this topic successfully is being aware of the misconceptions. Keep checking in on your students with regular diagnostic tasks and put strategies in place to help them make links between macroscopic observations, sub-microscopic processes, and how these can be represented symbolically in equation work. And remember, when life gives you lemons, make an electrochemical cell!
Louise Hussein teaches chemistry at an independent girls’ school in Dulwich, Greater London















No comments yet