Channel your students’ energy into mastering this tricky topic with these tips, ideas and classroom activities
Why do chemical reactions happen? Why did a warehouse of ammonium nitrate destroy a capital city in 2020? Why did the London fire brigade conclude that a fire started when washing spontaneously combusted? Thermodynamics provides the answers. Asking these types of context-based questions is a great way to start a discussion with your students about the feasibility of reactions, entropy and free energy.
Why do chemical reactions happen? Why did a warehouse of ammonium nitrate destroy a capital city in 2020? Why did the London fire brigade conclude that a fire started when washing spontaneously combusted? Thermodynamics provides the answers. Asking these types of context-based questions is a great way to start a discussion with your students about the feasibility of reactions, entropy and free energy.
Download this
Spot the errors, for age range 16–18
Challenge your post-16 learners to identify and correct mistakes in entropy calculations and explanations’.
Download the student sheet, teacher notes and slides from the Education in Chemistry website: rsc.li/3B3cDCU
Entropy and entropy change are abstract ideas used to explain why changes occur in the direction that we observe under specific conditions. Entropy is often described as a measure of disorder of a system, but it is actually a thermodynamic property that can be used to determine the energy not available for work in the process.
What students need to know
- Entropy, S, is a measure of the number of ways of arranging particles and energy in a system.
- The units are J mol-1 K-1.
- S (gas) > S (liquid) > S (solid)
- The entropies of more complex molecules are larger than those of simple molecules.
- A change in the number of particles as a result of a reaction will affect the entropy of the system.
- The standard entropy change of a reaction can be calculated from the standard entropies of reactants and products.
- Entropy calculations include:
- ∆Stotal = ∆Ssystem + ∆Ssurroundings
- ∆Ssurroundings = –∆H/T
- ∆Stotal = ∆Ssystem –∆H/T
- ΔG = ΔH – TΔS (where ΔG is Gibbs free energy)
- Spontaneous reactions occur when ∆Stotal is positive and ΔG is negative.
Common misconceptions
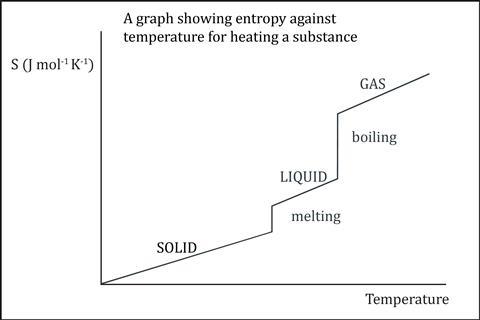
Learning about abstract concepts is challenging because students cannot see or measure any properties. Asking students to complete a sketch graph of entropy against temperature will help to bolster the idea that entropy is a property and will reveal any underlying misconceptions. Students often incorrectly draw horizontal lines to indicate entropy in the solid, liquid or gaseous state. If the temperature rises, then the vibration of the particles of the substance, and thus the entropy, will increase.
The terms enthalpy and entropy can at first seem similar but are, in fact, very different. Key difficulties are:
- using the correct equation;
- recalling standard temperatures; and
- confusing units by failing to convert temperature into kelvin or using inconsistent energy units, particularly when calculating the entropy change of surroundings – ∆Ssurroundings = –∆H/T.
A good starting point is to look at the question and identify:
- what I know now;
- what I can work out immediately; and
- what I’ve been asked for.
The terms enthalpy and entropy can at first seem similar but are, in fact, very different. Key difficulties are: using the correct equation; recalling standard temperatures; and confusing units by failing to convert temperature into kelvin or using inconsistent energy units, particularly when calculating the entropy change of surroundings. A good starting point is to look at the question and identify: what I know now; what I can work out immediately; and what I’ve been asked for.
Ideas for your classroom
Before starting, ensure your students have a secure understanding of energy and change, including specialist language such as system and surroundings.
Before starting, ensure your students have a secure understanding of energy and change, including specialist language such as system and surroundings.

One way is to focus on order and disorder, starting with a familiar context. A simple way of demonstrating this is by arranging marbles of two different colours in two layers in a large plastic beaker, and then shake it. Ask students how likely it is that the system will return to the original ordered arrangement. It’s all about probability. Next, move the conversation on to thinking about chemical events – for example, the odds of two chemicals mixing are very high if both have similar types of molecules.
Then explore why chemical reactions happen. Introduce the idea that entropy can give accurate predictions about how a particular system will behave, but not what the individual atoms will do. You’ll find more detailed explanations and further examples in Chemistry is like a crowd and these post-16 thermodynamics tutorials. The sequence of activities in this lesson plan looking at why chemical reactions happen provides a structured approach, through group discussions, demonstrations and role play, to understanding the question: why do chemical reactions occur?
Then explore why chemical reactions happen. Introduce the idea that entropy can give accurate predictions about how a particular system will behave, but not what the individual atoms will do. More detailed explanations and further examples can be found in Chemistry is like a crowd (rsc.li/3OPMJrY) and the Quantum casino tutorial (rsc.li/3ujpddr). The sequence of activities in this lesson plan looking at why chemical reactions happen (rsc.li/3FfUyUE) provides a structured approach, through group discussions, demonstrations and role play, to understanding the question: why do chemical reactions occur?
More resources
- Display the Real-life contexts for thermodynamics and Born–Haber cycle infographics in your class. Use the accompanying worksheets and interactive Born–Haber toolkit with your 16–18 learners to practise calculations and drawing cycles.
- Apply understanding of enthalpy change, structure and bonding with exam-style questions based on the Beirut explosion and ammonium nitrate in the Runaway reactions resource.
- Show students this video job profile of project leader, Stuart McDonald, and highlight the importance of chemistry in research and development.
- Assess your learners’ understanding of key definitions, entropy, Gibbs free energy and more with the Thermodynamics starter for 10.
After having secured the qualitative aspects of entropy, consider the numerical value of different substances. Provide students with the data in the table, on the entropy of a range of different substances, and ask them to write a set of statements, such as diamond has a very low entropy because it forms a highly structured giant covalent molecule. This activity leads nicely into calculating the entropy change for a reaction (or system).
| Substance | S (J mol-1 K-1) |
|---|---|
|
C (diamond) |
2 |
|
C (graphite) |
6 |
|
NaCl (s) |
72 |
|
H2O (s) |
44.8 |
|
H2O (l) |
70 |
|
H2O (g) |
189 |
|
CH4 (g) |
186 |
|
CO2 (g) |
214 |
Further develop the idea of entropy using the progression of ideas (see below panel) to sequence student learning. Finally, introduce the question of feasibility and whether a reaction will occur. Here, we need to bring together and build on previous learning as we consider the relationship between enthalpy change, entropy change and temperature and the idea of free energy: ΔG = ΔH – TΔS.
- If ΔG is negative, the reaction is spontaneous.
- If ΔG is zero, a reaction is in equilibrium.
- If ΔG is positive, the reaction is not spontaneous.
This tutorial on Gibbs free energy provides background reading and examples. And this activity on entropy and equilibrium uses models to develop some of the ideas provided.
Further develop the idea of entropy using the progression of ideas (see below panel) to sequence student learning. Finally, introduce the question of feasibility and whether a reaction will occur. Here, we need to bring together and build on previous learning as we consider the relationship between enthalpy change, entropy change and temperature and the idea of free energy: ΔG = ΔH – TΔS. If ΔG is negative, the reaction is spontaneous. If ΔG is zero, a reaction is in equilibrium. If ΔG is positive, the reaction is not spontaneous.
This tutorial on Gibbs free energy (sc.li/3gRwH4i) provides background reading and examples. And this activity on entropy and equilibrium (rsc.li/3Vn9vd9) uses models to develop some of the ideas provided.
The progression of ideas
Use these ideas to develop student understanding of entropy in this sequence:
- Order and disorder
- Probability and chance in everyday life
- What entropy means
- Entropy change of the system
- Entropy change of the surroundings
- Total entropy change
- Second law of thermodynamics
- Measuring enthalpy change
- Gibbs free energy
- Free energy and the enthalpy change of the system
- Free energy and temperature
- Free energy and spontaneous reactions
- Free energy and reversible reactions
Checking students’ understanding
Throughout this topic, use formative assessment to gain insight into how your students think. You could use these tips on making the most of formative assessment.
Regularly check basic qualitative understanding of entropy, using quick-fire low stake questions, such as: how does the entropy change in NaCl(s) → NaCl(aq)? To reveal more about your students’ understanding, ask them to model the changes in the arrangement of particles during the process. Further examples can be found in this thermodynamics resource. The tremendous thermodynamic poster provides further teaching contexts.
Regularly check basic qualitative understanding of entropy, using quick-fire low stake questions, such as: how does the entropy change in NaCl(s) à NaCl(aq)? To reveal more about your students’ understanding, ask them to model the changes in the arrangement of particles during the process. Further examples can be found in this thermodynamics resource (rsc.li/3ORDyXS). The tremendous thermodynamic poster (rsc.li/3udHB7o)provides further teaching contexts.
This is a great opportunity to pick up on examiners’ comments, and you can use the resource download with this article to do so.
Take-home points
- Be upfront – entropy is a difficult concept due to its abstract nature.
- Introduce the topic using everyday examples to get discussion started.
- Carefully sequence learning to support conceptual understanding. Ensure your students have a good qualitative understanding of entropy and can apply the ideas to a range of different systems before moving on to quantitative calculations.
- Regularly use formative assessment to monitor progress and student understanding.
Dorothy Warren is an independent science education consultant based in the UK














1 Reader's comment