Peter Banks suggest ideas, activities and resources for your classroom
Because students familiar with the table’s development and structure make better progress
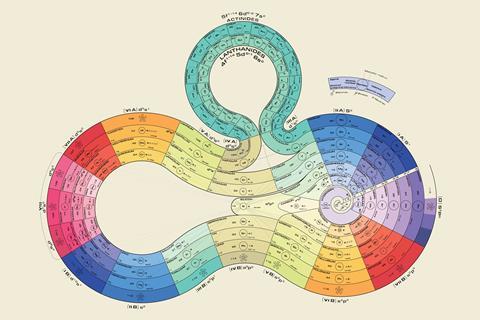
Dmitri Mendeleev’s periodic table is truly iconic. In the 150 years since its conception it has become the chemistry equivalent of the alphabet. While getting students to memorise the elements by rote is unhelpful – although there’s always a couple of students who think that memorising it makes them master chemists, understanding how the period table is structured is one of the most important concepts that they’ll learn in our labs.
Dmitri Mendeleev’s periodic table is truly iconic. In the 150 years since its conception it has become the chemistry equivalent of the alphabet. While getting students to memorise the elements by rote is unhelpful – although there’s always a couple of students who think that memorising it makes them master chemists, understanding how the period table is structured (rsc.li/2o891gG) is one of the most important concepts that they’ll learn in our labs.
Weaving the human stories behind the periodic table into our teaching can help with this. Hearing tales of the early attempts at constructing a periodic table in the 1860s, and about the endeavours of those smashing atoms together today in the quest to extend the periodic table, will also give students an appreciation of how science really works.
Weaving the human stories behind the periodic table into our teaching can help with this. Hearing tales of the early attempts at constructing a periodic table in the 1860s, and about the endeavours of those smashing atoms together today (rsc.li/35P4bG2) in the quest to extend the periodic table, will also give students an appreciation of how science really works.
What students need to know
- Chemists have been trying to order the elements since they were first discovered.
- Patterns in the periodic table help chemists to predict properties of elements.
- The periodic table is a live document, updated and improved as new discoveries are made and evidence found.
- There are parallels in the development of the periodic table and the development of our understanding of the atom.
- Scientific ideas can change – models are modified as new evidence is found.
Ideas for your classroom
Take your students back in time to the 1860s and give them some of the information about the elements that the mid-19th century chemists would have had access to. Ask them to use this to arrange and organise the elements into a logical grid structure. How do your students choose to group the elements? How are they ordering them? What does their table look like?
Download this
A template to make your own Elements Top Trumps (as MS Word or pdf) and a slide show of some early periodic tables (as MS Powerpoint and pdf).
When they have developed their model, wind time forward and provide some of the new evidence about elements that we have today. (It may be helpful to avoid the transition elements for now.) Does this change their model? Ask them to justify their arrangements and groupings. The Elements Top Trumps card game is useful here, as is the RSC interactive periodic table.
When they have developed their model, wind time forward and provide some of the new evidence about elements that we have today. (It may be helpful to avoid the transition elements for now.) Does this change their model? Ask them to justify their arrangements and groupings. The Elements Top Trumps card game (available from Amazon amzn.to/2P76TAI) is useful here, as is the RSC interactive periodic table (rsc.li/35LYnND).
When your students have decided on their periodic table design and explained their reasoning, introduce them to the early periodic table attempts that lead up to Mendeleev’s first periodic table. Next provide details of the noble gases, which were discovered after Mendeleev’s first table, and show them how they perfectly fit in their place.
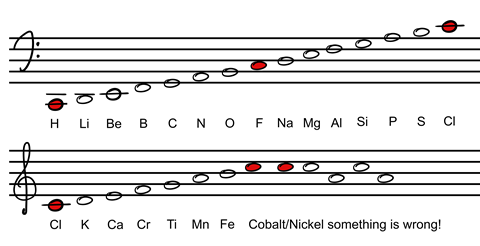
When your students have decided on their periodic table design and explained their reasoning, introduce them to the early periodic table attempts (rsc.li/31A4TU6) that lead up to Mendeleev’s first periodic table. Next provide details of the noble gases, which were discovered after Mendeleev’s first table, and show them how they perfectly fit in their place.
Common misconceptions
It is common for students to think that the periodic table has remained unchanged since the 1800s. Discussing recent additions can help with this. The newest elements on the periodic table are nihonium (113), moscovium (115), tennessine (117) and oganesson (118). These were all added in 2016.
Take care with how you define an element when covering these recent additions. Students are often told that ‘elements cannot be made’ or ‘elements are always there’. But when we talk about adding new elements to the periodic table, these definitions can cause confusion. The new elements are synthesised in nuclear reactors or particle accelerators, processes that are very different from the formation of new substances in chemical reactions. Until students discover the distinct differences between chemical and nuclear reactions at 14–16 they can find this concept challenging. I use the term ‘discovering’ and avoid any mention of ‘making’ elements to counter this.
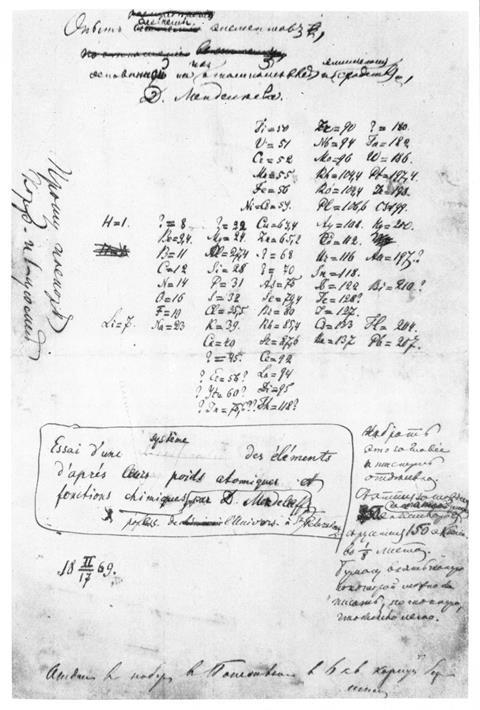
Teaching of the history of the periodic table offers an opportunity to recap many important points within chemistry and iron out common chemical misconceptions. Concepts that will be revisited here include atoms versus elements and molecules versus compounds.
Teaching of the history of the periodic table offers an opportunity to recap many important points within chemistry and iron out common chemical misconceptions. Concepts that will be revisited here include atoms versus elements and molecules versus compounds (rsc.li/31vA56X).
Many students find it hard to grasp the complexities behind the periodic table or to recognise that it is more than just an ordered list of the elements. Try showing them some of the patterns that lie hidden underneath the surface. This can be done in just a couple of minutes, for example by demonstrating the reactions of the group 1 alkali metals with water or displacement reactions of the group 7 halogens. If you’ve already covered electronic structure, you can also show them the number of electrons in the outer shells in each group and across each period.
Many students find it hard to grasp the complexities behind the periodic table or to recognise that it is more than just an ordered list of the elements. Try showing them some of the patterns that lie hidden underneath the surface. This can be done in just a couple of minutes, for example by demonstrating the reactions of the group 1 alkali metals with water (rsc.li/32yfJvr) or displacement reactions of the group 7 halogens (rsc.li/2qpkuZQ). If you’ve already covered electronic structure, you can also show them the number of electrons in the outer shells in each group and across each period.
There is no merit in memorising the entire periodic table, but do encourage familiarisation with the first 20 elements. While this won’t improve students’ chemical understanding, it will help them speed up finding elements on the table.
There is no merit in memorising the entire periodic table (rsc.li/31vyqhJ), but do encourage familiarisation with the first 20 elements. While this won’t improve students’ chemical understanding, it will help them speed up finding elements on the table.
Formative assessment
Start by asking students what they think is important about the periodic table. Find out what they know about its contents and build up their understanding from here. Issue your class with a variety of undated historical versions, and ask them to predict the order they were created in. Ask them to analyse the early periodic table designs – what are the advantages and disadvantages of each version? What changes or improvements need to be made to each version and why?
Progression 14–16
At 14–16, students will learn about patterns of reactivity and will use the periodic table to determine the number of shells and electrons in an atom. As they progress in their studies, they will also look at periodicity and find many more patterns. Students will also learn about the development of the atomic model, which has parallels in the scientific processes used by Mendeleev and others.
Take-home points
- The periodic table has evolved over time, changing as new evidence was collected and ideas developed.
- Elements in the modern age are synthesised in nuclear reactions and particle accelerators rather than discovered in nature – avoid in-depth discussion of this at 11–14.
- Give students an opportunity to develop their own ordering of the elements – model the processes used by Mendeleev.
- The processes of the development of the atomic model has parallels in the development of the periodic table.
Download this
A template to make your own Elements Top Trumps and a slide show of some early periodic tables from the Education in Chemistry website: rsc.li/xxxxxxx













1 Reader's comment