Create chemical gardens with your students by growing crystals of coloured silicates in this class practical
In this experiment, students add crystals of various metal salts to a mixture of sodium silicate and water in a beaker. After leaving the beaker overnight, students can observe crystals of coloured silicates formed by precipitation of the metal ions with silicate ions, producing their own chemical ‘garden’.
This is not only a good end-of-term experiment to let students grow crystals, which they find a fascinating process in itself, but is also a useful means of illustrating the appropriate chemistry involved when teaching introductory Earth science. The formation of molten silicates in the Earth’s mantle involves the reaction of silicon dioxide with metal oxides at extremely high temperatures.
This is a very straightforward experiment and can be carried out easily by groups of two in 30 minutes. The crystals start to develop overnight, but the experiment can be left for several days or for more than a week, with perhaps a competition being held to judge which is the finest ‘garden’.
Give this experiment an artistic context
Invite students to imagine themselves as ancient Roman science-artists investigating crystals using a version of this practical, suitable for 11–14 year olds, created as part of our Chemistry and art collection.
Download the student handout and teacher notes below.
Download the student handout
Download the teacher notes
Equipment
Apparatus
- Eye protection (goggles) for handling the sodium silicate solution
- Disposable gloves (preferably nitrile)
- Beaker, 500 cm3
- Watch glass
- Glass stirring rod
- Forceps
- A piece of card, to cover the beaker
Chemicals
- Sodium silicate solution (water glass) (CORROSIVE) (see note 3 below)
- A few crystals of some metal sulfates or nitrates (see note 8), such as:
- Cobalt(II) nitrate (OXIDISING, HARMFUL) (see note 9)
- Iron(III) nitrate (OXIDISING, IRRITANT)
- Magnesium nitrate (OXIDISING)
- Manganese(II) sulfate (HARMFUL, DANGEROUS FOR THE ENVIRONMENT)
- Hot deionised water
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection (goggles) and disposable gloves throughout. Remember to handle the crystals only with a pair of forceps. Do not use your fingers.
- Sodium silicate (water glass) solution (CORROSIVE) – see CLEAPSS Hazcard HC095B and CLEAPSS Recipe Book RB000. Sodium silicate is supplied in solution as an egg preservative. This type of solution is ideal for these experiments, as it is very difficult to dissolve the solid.
- Cobalt(II) nitrate, Co(NO3)2.6H2O(s) (OXIDISING, HARMFUL) – see CLEAPSS Hazcard HC025 AND note 9 below.
- Iron(III) nitrate, Fe(NO)3.9H2O(s), (OXIDISING, IRRITANT) – see CLEAPSS Hazcard HC055C.
- Magnesium nitrate, Mg(NO3)2.6H2O(s), (OXIDISING) – see CLEAPSS Hazcard HC059b.
- Manganese(II) sulfate, MnSO4.7H2O(s), (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC060.
- The metal salts chosen are known to be reasonably soluble in water. If a particular metal compound is unavailable, a nitrate is usually a safe choice as an alternative, or even the chloride if a Data Book indicates that the solubility is as high as that of the nitrate or sulfate.
- Nickel and cobalt compounds are CARCINOGENIC and SENSITISERS (and more). They should not be used by 11–14 year old pupils. If it is required, the teacher can set up a demonstration garden with these.
- Powders of the chemicals can be used if crystals are unavailable. The powder can be carefully dropped against the side of the beaker just above the water line. If any floats on the surface it can be nudged under the water to drop to the bottom of the beaker. The powders still produce an interesting array of crystal tendrils.
Procedure
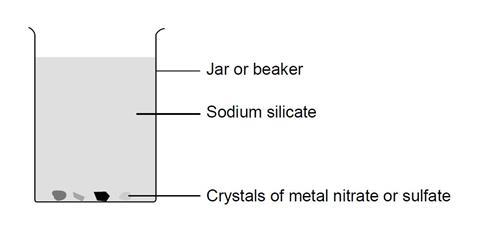
- Pour sodium silicate solution (CORROSIVE – wear goggles) into the beaker to a depth of about 3 cm.
- Add hot deionised water to this solution, stirring well with a glass rod, until the final depth is about 12 cm.
- Continue stirring until the sodium silicate and water are thoroughly mixed, and no separate layers are visible.
- Allow the mixture to stand until the liquid is completely still.
- Use a pair of forceps to drop one or two crystals of each of the metal salts supplied into the mixture. Try to ensure that the crystals do not fall close to each other.
- Cover the beaker with a piece of card and leave overnight.
Teaching notes
There is a great temptation for students to want to handle the crystals, especially when these are not properly held by the forceps and drop before entering the beaker. Gloves can be avoided if students are careful to use forceps.
The very best effects are observed when students use a relatively small number of crystals and arrange these in a well-separated manner at the bottom of the beaker.
For more able students it may be appropriate the explain that:
- The metal ions are mostly chosen from the d-block of the periodic table (these may be better known to students as the transition metals), since it is these which are coloured.
- The reaction taking place is a precipitation of the metal ions with silicate ions. A simplified equation for the reactions taking place:
- eg cobalt(II) ions from the metal salt and silicate ions from the sodium silicate solution form insoluble cobalt(II) silicate:
Co2+(aq) + SiO32–(aq) → CoSiO3(s)
- eg cobalt(II) ions from the metal salt and silicate ions from the sodium silicate solution form insoluble cobalt(II) silicate:
Obviously whereas the reaction occurring in the laboratory is taking place in solution, the analogous process taking place in the Earth’s crust involves ions in a molten state at extremely high temperatures linking together.
Downloads
Making a crystal garden - student handout
Editable handout | Word, Size 76.84 kbMaking a crystal garden - student handout
Handout | PDF, Size 0.18 mbMaking a crystal garden - teacher notes
Editable handout | Word, Size 42.75 kbMaking a crystal garden - teacher notes
Handout | PDF, Size 0.13 mb
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry

















No comments yet