A question and answer sheet to test learner’s knowledge of polymers.
The topics covered in this Starter for ten activity are: common polymers, condensation polymers, and poly(alkenes).
Example questions
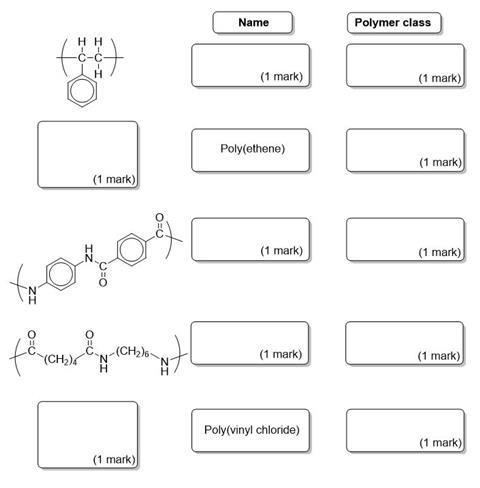
Complete the diagram showing the structures, common names and class of polymer (addition or condensation) for the polymers shown.
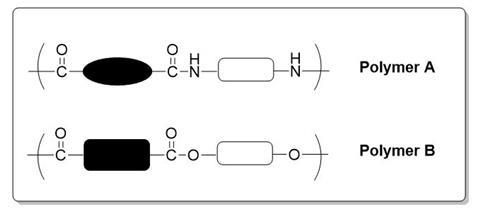
1. The box above shows the repeating units of 2 polymers A and B
Draw the monomers required for the production of each polymer.
State the general name given to each polymer based on the functional groups and bonding they contain.
What small molecule is produced during both polymerisations?
2. Nylon (6,6) can be formed from the polymerisation of the monomers hexane-1,6-dioylchloride and 1,6 diaminohexane. Draw the repeating unit of Nylon (6,6).
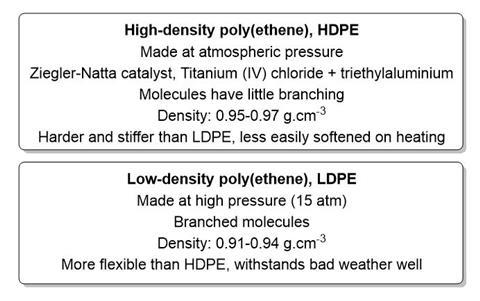
Poly(ethene) was discovered by accident. The chemical company ICI was carrying out research into new dyes in the 1930s when two chemists Eric Fawcett and Reginald Gibson accidently synthesised this new polymer. They were studying the reaction between ethene and benzaldehyde under a pressure of 2000atm; they hoped to make a ketone. The reaction was left to run over the weekend and when some ethene was lost due to leakage they added more ethene. When the reaction vessel was opened, a white waxy solid was found and on analysis it was shown to have the empirical formula CH2. They called it polyethylene as ethylene was the traditional name for ethene. The synthesis was repeated several times, sometimes successfully and sometimes with explosions. Because of the risky nature of the synthesis, development was stopped in 1933 but by 1935 engineers had constructed a reactor vessel that could withstand high pressure and work recommenced in December 1935. Controlling the pressure allowed the chemists to control the molar mass of the polymer and after one month the researchers had produced enough material to show that it could be moulded and was an electrical insulator. The first poly(ethene) products appeared in shops in 1948. Today we commonly use two types of poly(ethene) as described below.
- Write an equation for the reaction the scientists were trying to achieve when they discovered poly(ethene)
- State the repeating unit of poly(ethene)
- Why were explosions a hazard of the operating conditions?
- What property of LDPE makes it suitable for food packaging?
- With reference to the structure and bonding in LDPE and HDPE, explain why HDPE has a higher melting point
- Give the formulae of the component chemicals of the Ziegler-Natta catalyst.
- Poly(alkenes) have replaced the use of natural rubber in many cases. The monomer of natural rubber is 2-methylbuta-1,3-diene. Draw the displayed formula of this monomer.
Notes
A full version of this question and answer sheet is available from the ‘Downloads’ section below. An editable version is also available.
Downloads
Polymers - editable
Word, Size 0.3 mbPolymers
PDF, Size 0.29 mb
Starters for 10: Advanced level 2 (16–18)
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
 Currently reading
Currently readingPolymers
- 9
- 10
- 11
- 12
- 13
- 14
- 15































No comments yet