A question and answer sheet to test learners’ knowledge of compounds with amine groups
The topics covered in this Starter for ten activity are: classifying amines and amides, properties of amines, amine mechanisms, amino acids, amino acids and pH, and amine preparation.
Example questions
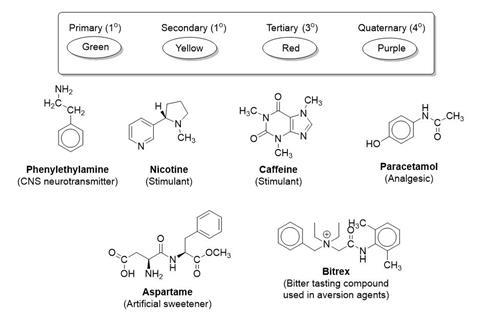
Amines are compounds containing a basic nitrogen atom with a lone pair. They are derivatives of ammonia where bonds are formed with one or more organic ‘R’ groups. When the lone pair is also used in co-ordinate bonding with say a H+ ion then the compound formed is called a quaternary ammonium salt. When an amino group is next immediately adjacent to a carbonyl C=O group, the compounds are called amides. Both types of compound can be classified as primary (1°), secondary (2°), tertiary (3°) or quaternary (4°), according to the number of organic R groups on the nitrogen.
Look at the following chemicals containing amine and amide groups. Colour code the groups according to their class.
Oliver took some ammonia solution (Beaker A) and added some dilute HCl. Before the addition the ammonia had a distinctive ‘fishy’ odour. Following the addition this went away (Beaker B). When dilute NaOH was added to beaker B the fishy odour returned.
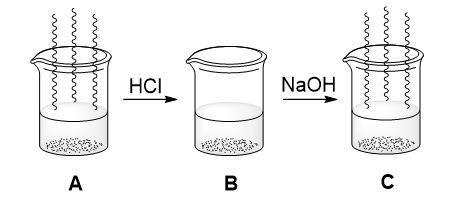
- Using your knowledge of amine chemistry, explain the observations Oliver made.
- George took some ammonia and added it to water. He added universal indicator to the solution which turned purple. Explain these observations.
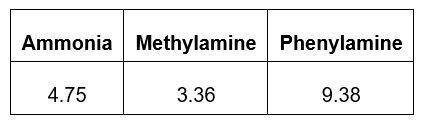
- The table below shows the pKa values for some amines. The smaller the pKa value, the stronger the base. Discuss these values.
Notes
A full version of this question and answer sheet is available from the ‘Downloads’ section below. An editable version is also available.
Downloads
Compounds with amine groups - editable
Word, Size 0.49 mbCompounds with amine groups
PDF, Size 0.46 mb
Starters for 10: Advanced level 2 (16–18)

This chapter in our Starters for ten series covers: kinetics, equilibria, acids and bases, carbonyl chemistry, aromatic chemistry, compounds with amine groups, polymers, structure determination, organic synthesis, thermodynamics, periodicity, redox equilibria, transition metal chemistry, and inorganics in aqueous solution.
- 1
- 2
- 3
- 4
- 5
- 6
 Currently
reading
Currently
reading
Compounds with amine groups
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15















































1 Reader's comment