Test learners knowledge of aromatic chemistry
The topics covered in this Starter for ten activity are: Naming aromatic compounds, industrially important molecules. structure of benzene, electrophilic substitution, and synthestic routes with benzene.
Example questions
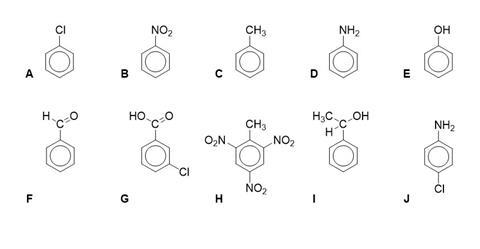
Name the following aromatic derivatives using IUPAC nomenclature
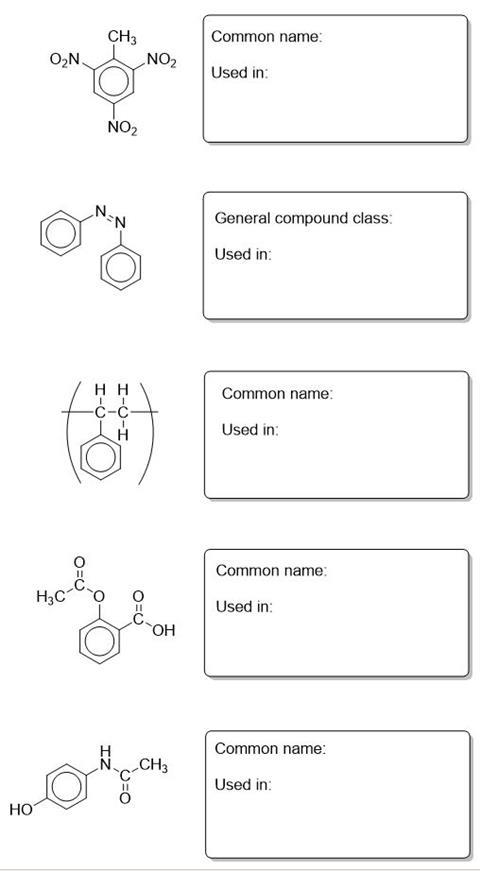
Name the benzene derivative and outline why it is industrially important.
The benzene ring is present in many important compounds and therefore determining its structure was very important. The German chemist Kekulé (1865) had suggested that benzene existed as a hexagonal carbon ring with alternate single and double bonds. Professor Ingold of London University prepared some crystals of hexamethylbenzene and sent them to the crystallographers at the University of Leeds for analysis. There Kathleen Lonsdale showed by mathematical analysis of her X-ray diffraction pattern that Kekulé’s proposed structure was incorrect. Kathleen later became a Professor at University College London and only the second woman fellow of the Royal Society.
- Draw the structure Kekulé proposed
- Why could the crystallographers not work on benzene rather than the hexamethyl derivative?
- If Kekulé’s structure were true, what would the X-ray of benzene show? (you can use pictures to illustrate your answer where appropriate)
- What features of benzene were shown up by the X-ray analysis that helped to disprove the Kekulé structure?
- If benzene had the structure proposed by Kekulé, what kind of reaction mechanisms would it show?
The enthalpy of hydrogenation of cyclohexene is -120 kJ.mol-1. Using this information what would you expect the enthalpy of hydrogenation of benzene to be if the Kekulé structure holds true? The actual enthalpy of hydrogenation of benzene is -208 kJ.mol-1. What does this infer about the actual structure of benzene?
Notes
The full question and answer sheet is available from the ‘Downloads’ section below. An editable version is also available.
Downloads
Aromatic
PDF, Size 0.32 mbAromatic - editable
Word, Size 0.34 mb
Starters for 10: Advanced level 2 (16–18)

This chapter in our Starters for ten series covers: kinetics, equilibria, acids and bases, carbonyl chemistry, aromatic chemistry, compounds with amine groups, polymers, structure determination, organic synthesis, thermodynamics, periodicity, redox equilibria, transition metal chemistry, and inorganics in aqueous solution.
















































No comments yet