Watch this
Watch the video and download the technician notes from the Education in Chemistry website: rsc/li/xxxxxxx
Last year, Bob Worley from CLEAPSS delivered one of his fantastic microscale workshops at our school to teachers from around our region. One demonstration he was particularly keen on showing off was the hydrogenation of propene, based on a method developed by Bruce Mattson at Creighton University in the US. We used Mattson’s technique for producing chlorine gas samples in syringes in a recent Exhibition chemistry – and here we are employing one of his ‘catalyst tubes’ to quickly and easily hydrogenate an alkene without the need for heating.
Kit
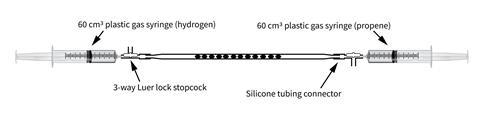
- 60 cm3 plastic syringes loaded with 30 cm3 of gaseous alkene (such as propene)
- 60 cm3 plastic syringe loaded with approx 35 cm3 of hydrogen
- Silicone tubing
- Glass Pasteur pipette
- Two clamp stands with clamps
- 0.002 M acidified potassium manganate(VII) solution, approx 1 cm3
- 0.002 M bromine solution, approx 1 cm3
- Small sample vials
- Sticky tack or two 3-way Luer-lock stopcocks
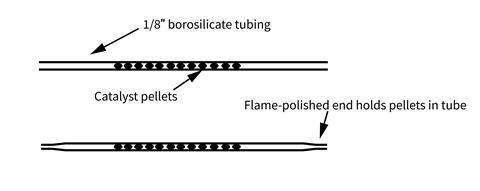
- Reusable catalyst tube made from approx 15 cm of 1/8 inch (3.2 mm) internal diameter borosilicate glass tubing, or palladium on alumina, 0.5 wt. %, 3.2 mm pellets, 0.5 g
Preparation
The catalyst tube can be produced by flame-polishing one end of the glass tube to constrict the opening slightly. You only need to do this once as you can reuse the tube. Use forceps to avoid handling the palladium pellets. Drop approx 0.5 g of them into the opposite, wider end of the tube to trap them in a line 4–6 cm long. With the pellets gathered at the constricted end of the tube, the wider end of the tube can now be flame-polished to permanently hold the pellets within. They should be able to move freely within the tube (so as to not block the passage of gases), but not fall out of either end. Finally place 1–2 cm of silicone tubing on each end for connecting with the syringes.
If the pellets you acquire are brown in colour, before first use they may need activating by rotating the tube in a Bunsen heating flame until they turn dark grey/black.
Similarly, a hydrogen syringe can be preloaded from a cylinder or produced in situ using the Mattson technique illustrated in the January 2019 Exhibition Chemistry (also see his free online book Microscale Gas Chemistry, chapter 3). Gases can be sealed inside with either a syringe cap or sticky tack.
In front of the class
Work well away (> 1 m) from any naked flames. Clamp the two syringes facing one another and attach the catalyst tube to the end of the hydrogen syringe. Flush through approx 5 cm3 of hydrogen to clear any air from the catalyst tube before attaching the other end to an alkene syringe. 3-way Luer-lock stopcock valves are ideal for this but sticky tack gives a good seal too.
Point out that both syringes read 30 cm3, for a total of 60 cm3 of gas, and use the plungers to pass gas over the catalyst from one syringe to another. The catalyst tube will feel warm to the touch and the total volume of the gases will decrease. With 100% yield, the total volume will drop to 30 cm3, demonstrating Avogadro’s law as applied to equation 1. However yields will vary from upwards of 50% depending on the quality of both the catalyst and alkene mix.
Equation 1: C3H6 + H2 → C3H8
Bubble a few cm3 of product gas into approx 1 cm3 of 0.002 M acidified potassium manganate(VII) solution or 0.002 M bromine solution in a small sample vial, close the lid and shake. The product gas will not decolourise the contents, while repeating with the starting alkene will.
Finally, fit some silicone tubing to a glass Pasteur pipette and fix the other end of the tubing to the gas syringe. Burn off the contents by igniting the end of the Pasteur pipette as you expel the contents of the syringe. The starting alkene will produce a smoky flame, while the hydrogenated gas will burn cleanly. The explanation for this was discussed in the March 2017 issue of Education in Chemistry.
If time allows, the procedure can be repeated with syringes containing 20 cm3 of acetylene–ethyne and 40 cm3 of hydrogen (after flushing the catalyst tube) – the volume drops towards 20 cm3 in line with Equation 2.
Equation 2: C2H2 + 2H2 → C2H6
Tips
Mattson’s original method suggests optimising the yield of the reaction by heating the catalyst tube in a Bunsen while 60 cm3 of hydrogen is flushed through just before attaching the reacting syringes. Due to the ignition source, this should only be done in a fume cupboard with the sash lowered to minimise risk of, and protect from, explosion.
A note on the catalyst
The 50 g bottle bought for this demonstration is currently available for approx £85 but contains enough pellets for 100 catalyst tubes. These can be used indefinitely and for numerous other reactions detailed in chapter 18 of Bruce Mattson’s book. Local schools may wish to do a group purchase if the initial outlay is prohibitively expensive.
Declan Fleming
Health and safety and disposal
See Cracking! from the November 2019 issue. Wear eye protection. All syringes and catalyst tubes can be kept for reuse. The product solution from the bromine water and acidified potassium manganate(VII) can be washed down the sink.
A fume cupboard is not needed for the propene reaction, but would be for the reaction of ethyne, or if the catalyst is heated.
Downloads
Technician notes
Word, Size 69.23 kbTechnician notes
PDF, Size 93.39 kb





















No comments yet