Watch this
Watch the video on the Education in Chemistry website: rsc.li/xxxxxxx
As I write this month’s Exhibition chemistry from the depths of a Covid-19 lockdown, I’m getting very used to the inside of my own flat. However, that doesn’t mean there aren’t plenty of interesting chemical phenomena worthy of exploration using only items around the kitchen – so many in fact that I decided to narrow it down and make use of the fruit we’re all hopefully eating to keep healthy. I have a good stock of citrus fruits and want to show a few little tricks you can do besides just turning them into clocks.
Demo 1: Flame thrower
Kit
- Orange
- Knife
- Candle
In front of the class
Cut off a piece of peel from an orange (3–5 cm in length and 2–3 cm wide is ideal), cutting as deep as possible without removing any juicy flesh. Heat the peel over a lit candle for a few seconds and then hold the warm peel, pointing the outer side of the peel towards the side of the flame, and firmly squeeze the top and bottom together to fold it in half. A burst of flame will shoot 5–10 cm from the candle and a puff of thick black soot will be released.
Teaching goal
The peel consists of two layers: the colourless, spongy inner albedo layer and the outer, coloured flavedo layer, which is rich in oil sacs containing peel oils. A major component of this surprisingly flammable oil is D-limonene. The unsaturated monoterpene is produced from two isoprene units – the building blocks of a range of chemicals familiar to students including menthol and R-carvone (mint and spearmint), oestrogen and testosterone, and the carotenoids which give many plants their yellow or orange colouration.
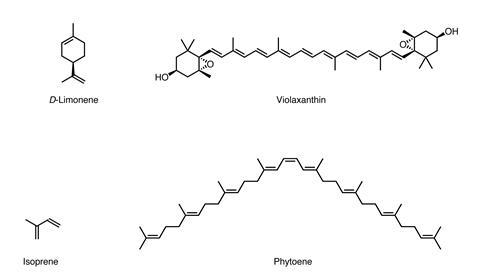
The flavedo of an orange is rich in carotenoids (eg violaxanthin) which, with their extended delocalised pi-system, are able to absorb blue light and appear orange. A lemon’s paler yellow flavedo, meanwhile, has a lower concentration of carotenoids. In this mixture there is also a much higher proportion of phytoene which, with less extensive delocalisation than other carotenoids, absorbs in the UV rather than the visible part of the EM spectrum, appearing colourless.
These heavily unsaturated compounds burn with a characteristically smoky flame – the explanation is explored in the article Clearing the air around smoke formation.
These heavily unsaturated compounds burn with a characteristically smoky flame – the explanation is explored in the article, ‘Clearing the air around smoke formation’ (rsc.li/2M1DFjW).
Back in the lab
Once you return to school, you can show the smoky and clean-burning flames of alkenes and alkanes with the cracking and hydrogenation demonstrations.
Once you return to school, you can show the smoky and clean-burning flames of alkenes and alkanes with the cracking and hydrogenation demonstrations (rsc.li/3d6kDox and rsc.li/2ZGikEO).
Demo 2: Balloon burster
Kit
- Orange
- Knife
- Candle
- Inflated balloon
In front of the class
Following the same procedure as above, cut a piece of orange peel and heat briefly over a candle flame to warm the oil sacs. This time, rather than squeezing the contents at a candle flame, try spraying the ejected oils at a well-inflated balloon or water balloon which, if taut enough, will pop.
Teaching goal
This demonstration shows how compounds with similar intermolecular forces are miscible. The balloon is made of latex, which is a polymer of isoprene, the base unit from which all terpenes are built. The terpene oils from the citrus peel therefore show similar intermolecular forces (in this case London dispersion forces) – so the oil can dissolve into the latex and disrupt the forces between polymer chains, causing the balloon’s structure to weaken. This is not a problem for water which, being dominated by hydrogen bonding interactions, does not degrade the latex.
Back in the lab
Once you return to school, you can show some other dissolving polymer effects. While it can take a few goes with orange oil to burst a balloon, the far more aggressive cyclohexane produces instantaneous and dramatic results.
Once you return to school, you can show some other dissolving polymer effects (rsc.li/2yybbeC). While it can take a few goes with orange oil to burst a balloon, the far more aggressive cyclohexane produces instantaneous and dramatic results.
Find out more
These demonstrations are based on a wider lab exploration of volatile fragrance molecules from Science in School. A microscale distillation set-up can allow students to extract a range of terpenes quickly and easily from plant matter, test for unsaturation, and assess the product’s purity with thin layer chromatography.
For more information on terpenes, use James Kennedy’s fantastic infographic, which illustrates the wide range of places in which they appear.
These demonstrations are based on a wider lab exploration of volatile fragrance molecules from Science in school (bit.ly/3grE6ly). A microscale distillation set-up can allow students to extract a range of terpenes quickly and easily from plant matter, test for unsaturation, and assess the product’s purity with thin layer chromatography.
For more information on terpenes, use James Kennedy’s fantastic infographic (bit.ly/2TBS5eU), which illustrates the wide range of places in which they appear.

























2 readers' comments