Supercharge your electrochemistry lessons with these misconception busters and teaching ideas
There are numerous articles and research papers about electrochemistry and its misconceptions. Why is this such a complex unit?
Students need to have a strong understanding of a wide range of topics for 14–16 electrochemistry, from structure and bonding to stoichiometry. It’s important to address misconceptions at this stage so students gain a solid understanding of A-level concepts.
Key misconceptions and Johnstone’s triangle
Misconceptions can arise in three key areas:
Misconceptions can arise in three key areas (bit.ly/3gkZyNy):
- One: identifying the anode and cathode
- Two: identifying products when aqueous or molten electrolytes are used
- Three: writing chemical equations
These issues are linked to the three parts of Johnstone’s triangle: macroscopic, sub-microscopic and symbolic, respectively.
Misconception one (macroscopic)
When trying to identify the anode and cathode, students often try to memorise positions of labels on diagrams instead of applying their knowledge. So they assign the labels incorrectly, because they don’t properly understand how the electrolytic cell diagrams are drawn, and they also don’t make the necessary links to practical observations.
Students need to know the symbol for an electrical cell, and how to identify the positive terminal on a cell/battery and a power pack. Some revision guides suggest using the mnemonic PANIC (Positive Anode Negative Is Cathode). However this can cause confusion at A-level when anode and cathode are assigned in galvanic cells.
It is better to describe the cathode as the cation acceptor, to help students understand what happens if the polarity of the cell is changed, and for comparison at A-level. I use the phrase cations go to the cathode when discussing electrolysis. This helps students correctly identify electrodes in tests. The RedCat mnemonic indicates that reduction occurs at the cathode.
When trying to identify the anode and cathode, students often try to memorise positions of labels on diagrams instead of applying their knowledge. So they assign the labels incorrectly, because they don’t properly understand how the electrolytic cell diagrams are drawn, and they also don’t make the necessary links to practical observations.
Students need to know the symbol for an electrical cell, and how to identify the positive terminal on a cell/battery and a power pack. Some revision guides suggest using the mnemonic PANIC (Positive Anode Negative Is Cathode). However this can cause confusion at A-level when anode and cathode are assigned in galvanic cells.
It is better to describe the cathode as the cation acceptor, to help students understand what happens if the polarity of the cell is changed, and for comparison at A-level. I use the phrase cations go to the cathode when discussing electrolysis. This helps students correctly identify electrodes in tests. The RedCat mnemonic indicates that reduction occurs at the cathode.
For example, the following reactions occur at the cathode …
- Electrolytic cell: NaCl(aq) 2H+ + 2e→H2
- Galvanic cell: Zn|Zn2+||Cu2+|Cu Cu2+ + 2e→ Cu
Misconception two (sub-microscopic)
Here, students need to be able to recognise aqueous solutions and molten liquids. They need to refer to the reactivity series and remember the rules for oxidation and reduction: oxidation is loss, reduction is gain (OILRIG). For positive ions in aqueous solutions, students should refer to the position of the metal ions relative to the hydrogen ions. If the metal ion is less reactive than the H+ ion, then the metal ion will be reduced. When considering negative ions, students also need to consider how dilute the solution is. Most GCSE specifications include the electrolysis of brine (concentrated NaCl) and the following rule helps: halide>hydroxide>other negative ions. In dilute solutions, hydroxide ions are oxidised in preference to halide ions and oxygen is discharged.
Modelling the sub-microscopic
The Education Endowment Foundation (EEF) Improving secondary science report suggests using modelling to improve understanding. Pedagogical tools like practicals can help students link the sub-microscopic to the macroscopic, and further support the symbolic (ie writing formula and equations), as described by Johnstone’s triangle.
Videos of modelling can help students build their understanding. Try this video about the chemistry observed in the electrolysis of brine practical from teacher Richard Butler. His use of labelled LEGO blocks supports the visualisation of ions in solution.
Microscale techniques support the discussion of electrolysis, and I use demonstrations to show how dissolved substances in distilled water result in electrical conductivity. We conduct a conductivity demo, where students can clearly see universal indicator colour changes in a small puddle of brine when electricity passes through it.
The Education Endowment Foundation (EEF) Improving secondary science report (bit.ly/3CGSJxC) suggests using modelling to improve understanding. Pedagogical tools like practicals can help students link the sub-microscopic to the macroscopic, and further support the symbolic (ie writing formula and equations), as described by Johnstone’s triangle.
Videos of modelling can help students build their understanding. Try this video about the chemistry observed in the electrolysis of brine practical from teacher Richard Butler (bit.ly/3VCl1BX). His use of labelled LEGO blocks supports the visualisation of ions in solution.
Microscale techniques support the discussion of electrolysis, and I use demonstrations to show how dissolved substances in distilled water result in electrical conductivity. We conduct a conductivity demo, where students can clearly see universal indicator colour changes in a small puddle of brine when electricity passes through it.
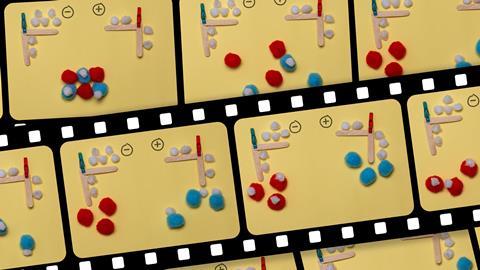
My colleague, Mrs Tricker, inspired me to use pompoms with our GCSE students, which led to successful modelling of electrolysis. I first set up an ‘ionic structure’ (photo 1) that dissolved (photo 2). Students had to predict direction of flow of the charged particles. We then linked this to the electrolysis of brine demonstration. We looked at how the electrons (white pompoms) were lost to the positive electrode, and gained from the negative electrode (cathode accepts cations), and how they flowed through the electrodes and wires (lolly sticks). Students wrote explanations of expected observations (photo 3) for an aqueous solution or molten liquid of their choice and made stop motion clips.

Misconception three (symbolic)
To write chemical equations students need to remember formulas of ions and balance half equations. They need to remember conventions for writing redox reactions, and use the correct terminology for explanations. This is where the modelling of ion movement enables students to construct half equations.
There are numerous practice questions available for students; Chemsheets resources are invaluable. You can also use past-paper questions and multiple-choice questions from the exam boards to check understanding and provide exam practice.
It’s important to use diagnostic questions to deal with misconceptions – the University of York BEST resources are excellent. And I found some useful diagnostic multiple choice questions from teacher Ms Keloglou on Twitter.
There are numerous practice questions available for students; Chemsheets resources (bit.ly/3TgrnWa) are invaluable. You can use Edexcel questions and multiple-choice questions from the CIE board to check understanding and provide exam practice.
It’s important to use diagnostic questions to deal with misconceptions – the University of York BEST resources are excellent. And I found some useful diagnostic multiple choice questions from teacher Ms Keloglou on Twitter (bit.ly/3s69woI).
Keep practising
Retrieval practice and spaced learning helps GCSE students pick up electrolysis concepts quickly and respond to questions with confidence. My students often link ideas to previous units, such as extraction of metals and testing gases. This is also the last topic we cover, so it’s easy to show connections to other aspects of the chemistry course.
More resources
- Discover how to improve students’ understanding with this article on common electrochemistry misconceptions.
- Find out how to minimise misconceptions through explanatory sequences.
- Use this practical video on the electolysis of aqueous solutions to help learners make links between the macroscopic and submicroscopic, with questions and activities in the supporting resources.
For A-level chemistry
- Help your students untangle common misconceptions about galvanic, electrolytic and concentration cells.
- Use number grids to help students understand electrochemistry.
- Tackle misconceptions when planning your lessons and curriculums with the RADAAR planning framework.















No comments yet