Demonstrate the movement of ions in a salt solution and make coloured writing using electricity and indicators
Students find this experiment electrifying, and their discoveries will spark curiosity in electrolysis.
This experiment should take 30-45 minutes.
Equipment
Apparatus
- Plastic petri dish
- Filter papers
- 6 V battery or power pack
- Leads and crocodile clips
- Carbon electrode
- Dropping pipette
Chemicals
- Sodium chloride
- Universal indicator
- Methyl orange
Health, safety and technical notes
- Read our standard health and safety guidance.
- Universal indicator is flammable, see CLEAPSS Hazcard HC032.
- For more information on sodium chloride, see CLEAPSS Hazcard HC047b.
- Use of electrical charges is always of risk, use protective equipment where the charge is high.
Procedure
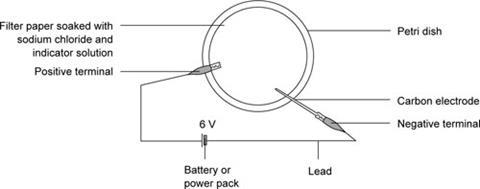
- Dissolve a spatula measure of sodium chloride in 2 cm3 of water. Add three drops of methyl orange indicator.
- Lay a filter paper inside a plastic petri dish. Drop the solution onto the paper using a dropping pipette, until the paper holds no more solution.
- Attach the positive end of a 6 V battery to a lead ending in a crocodile clip. Use the crocodile clip to grip one end of the paper.
- Attach the negative end of the battery to a carbon electrode.
- Write lightly on the wet paper, using the carbon electrode.
- Repeat the experiment using Universal Indicator.
Notes
Other indicators to try might include:
Bromocresol green (lead attached to positive terminal), screened methyl orange (try both terminals), blue litmus (positive) and red litmus (negative).
Phenolphthalein does not work very well in this experiment
When the ‘pencil’ is attached to the negative lead, H+ ions are attracted to it, producing the colour associated with acids for that particular indicator. If the ‘pencil’ is attached to the positive lead, the reverse happens.
Questions
- What would happen if the lead were attached to the positive electrode using universal indicator?
- Explain what reactions have occurred to produce the colours.
Answers
- When attached to the negative lead, the writing is red, when attached to the positive lead it is purple.
- H+ ions are attracted to the negative electrode, OH– ions are attracted to the positive electrode. So depending on which electrode the pencil is attached to it will affect the colour of the indicator and therefore the writing.
Downloads
Chemistry and electricity - student sheet
PDF, Size 0.15 mbChemistry and electricity - teacher notes
PDF, Size 0.13 mb
Additional information
This practical is part of our Classic chemistry experiments collection.


















No comments yet