Add some sparkle to your end-of-year lessons with this engaging, hands-on practical activity with a seasonal hook
Use this microscale practical experiment to teach about the important industrial process of electroplating. It can be used to revisit some of the key concepts of electrolysis and the follow up questions will help learners to consolidate their understanding of the processes.
This resource is part of a collection of ideas and activities for chemistry lessons in the festive season.
Introduction

Electroplating is the process of covering a metal object with a thin layer of another metal. The technique is often used to make cheap jewellery look expensive! For example, a ring made from a cheap metal like copper can be electroplated with either gold or silver. The result looks like a gold or silver ring without the high cost. Electroplating is also used to protect metals from corrosion. For example, the inside of a steel food can is electroplated with less reactive metal such as tin. The layer of tin provides a physical barrier protecting the steel from oxygen and water.
In this practical activity learners produce a ‘gold’ coin. First by electroplating a copper coin with zinc. Then by heating the plated coin in a flame to produce a brass layer giving it a ‘gold’ effect.
Learning objectives
1 Understand that electroplating is an important industrial application of electrolysis.
2 Electroplate a coin.
3 Describe how to electroplate a metal.
4 Explain what happens at each electrode during electroplating.
The introduction and experiment relate to learning objectives 1 and 2. The questions relate to all learning objectives.
Differentiation
The practical experiment is suitable for all learners within the 14–16 age range group. Some of the questions on the student sheet are more suitable for higher-level learners. Question 10 requires learners to produce a longer answer. A structure strip is given, providing scaffolded prompts to help overcome ‘fear of the blank page’. Use this long-answer question to consolidate learning. (Read more about how to use structure strips.) Removing the structure strip would increase the challenge of this question.
Equipment (per group)
- 6 V DC source or 9 V battery
- 2 x electrical leads
- 2 x crocodile clips
- 2 x steel paper clips
- Petri dish
- Measuring cylinder, 10 cm3
- Measuring cylinder, 25 cm3
- Beaker, 50 cm3
- Bunsen burner
- Heat proof mat
- Tongs
- Wash bottle or dropping pipette
- Cloth
- Copper coin (copper foil can be used if you do not have access to copper coins)
- Zinc foil
- Sodium hydroxide, 0.4 mol dm-3
- Zinc sulfate(VI), 0.1 mol dm-3
Safety equipment: safety spectacles
Method
Safety and hazards
- Wear safety glasses.
- Beware of sharp edges when manipulating the zinc foil.
- Take care not to get the electrolyte solutions on your skin.
- Work in a dry area. Make sure the power supply is switched off when you are putting the equipment together and that you switch it off again when you are dismantling the equipment.
- Hot coins can cause burns. Allow them to cool before you handle them.
Integrated instructions are available for this method.
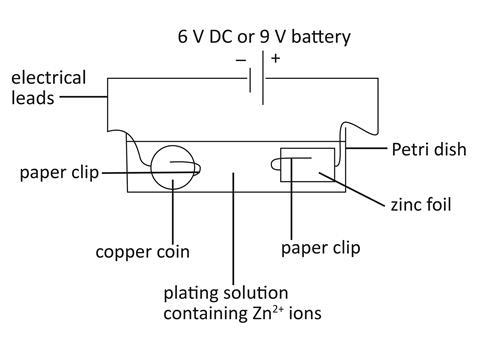
Set up the electroplating bath using a Petri dish and paper clips
- Straighten out the long ‘legs’ of the paper clips and place the coin and the zinc foil in the paper clip holders.
- Place both electrodes into the Petri dish.
- Attach the ‘long leg’ of the paper clip to a crocodile clip.
Make up the plating solution, an electrolyte containing Zn2+ ions
- Measure 12 cm3 sodium hydroxide (NaOH) and 2 cm3 zinc sulfate (ZnSO4) solutions using two different measuring cylinders.
- Add the sodium hydroxide and zinc sulfate solutions to a beaker.
- When all the white precipitate has dissolved pour the solution into the Petri dish.
Do the electroplating

- Using an electrical lead, connect the paper clip attached to the zinc to the positive connection of a 6 V DC power supply (or 9 V battery).
- Using an electrical lead, connect the paper clip attached to the copper coin to the negative connection of a 6 V DC power supply (or 9 V battery).
- When the coin looks ‘silver’, turn off the power and disconnect the wire from the paper clip holding the coin.
- Remove the coin with paper clip from the petri dish using tongs.
- Rinse the coin with water, then remove the paper clip holder.
- Dry the coin and polish it with a cloth.
Heat the coin
- Using tongs, gently pass the coin through a blue Bunsen burner flame until the coin looks golden.
- Place the coin on a heat proof mat and allow it to cool before handling.
Supporting chemistry
The electrolyte must contain the metal ions used for the plating. The best conditions for electroplating require a low concentration of the hydrated metal ion used for plating and a good conductivity in the solution (ie a high total concentration of ions). In this case, the zinc is in equilibrium but the position of the equilibrium lies well to the right, so the concentration of hydrated zinc ion, Zn2+(aq), is extremely low:
Zn2+(aq) + 4OH-(aq) ⇋ Zn(OH)4(aq)
Questions
The student sheet offers a set of follow-up questions to help learners consolidate their understanding of the chemistry. Answers are provided in the teacher notes.
More resources
- There are lots more microscale experiments, plus information and tips in our microscale chemistry collection.
- Use the animations in our video Electrolysis of aqueous solutions to illustrate the movement of ions in solution during the electrolysis of aqueous copper sulfate.
- The teacher demonstration Turning copper coins into ‘silver’ or ‘gold’ is a similar experiment and may be used to introduce the principles of electroplating before learners go on to electroplate their own coins on a microscale.
- CLEAPSS recommend an alternative method for electroplating copper with zinc on a reduced scale. You may feel that this method is more appropriate for some of your learners.
- Ancient coins, provides some background information about how coins have been part of our history for nearly 3000 years and the role chemistry plays in learning about the past! You may find some interesting snippets to drop into your lesson.
- Watch Trevor describe how his role as project manager at the world gold council helps to promote the value and uses of gold to different industries.
Downloads
Producing 'gold' coins microscale - teacher notes
Handout | PDF, Size 0.27 mbProducing 'gold' coins microscale - teacher notes
Editable handout | Word, Size 0.54 mbProducing 'gold' coins microscale - student instructions and worksheet
Handout | PDF, Size 0.35 mbProducing 'gold' coins microscale - student instructions and worksheet
Editable handout | Word, Size 0.58 mbProducing 'gold' coins microscale - technician notes
Handout | PDF, Size 0.32 mbProducing 'gold' coins microscale - technician notes
Editable handout | Word, Size 0.58 mbProducing 'gold' coins microscale - integrated instructions
Editable handout | PowerPoint, Size 0.51 mbProducing 'gold' coins microscale - integrated instructions
Handout | PDF, Size 0.47 mb



















No comments yet