Copper foil is folded into the shape of an envelope then heated with a Bunsen burner
When the foil has cooled, learners can open the envelope and discover that where there was no contact with oxygen the copper remained unreacted.
-

Download this
Download the scaffolded and unscaffolded student worksheets and teacher guidance (including answers) as MS Word and pdf and the presentation slides as MS Powerpoint and pdf.
Discover more resources from the Nuffield practical collection
The experiment will take about 20–30 minutes.
Learning objectives
- Safely heat copper using a Bunsen burner and record your observations.
- Describe and explain observations from a chemical reaction.
- Write word and symbol equations to represent a chemical reaction.
- Use simple calculations to link mass to reactivity and the availability of oxygen.
Success criteria
The practical allows learners to safely heat copper and record and discuss their observations (LO1 and LO2). Completing the follow-up questions using support from the PowerPoint will allow learners to succeed in LO2, LO3 and LO4.
Technician notes
Read our standard health and safety guidance and carry out a risk assessment before running any live practical.
Equipment
Apparatus
- Safety glasses
- Bunsen burner
- Heat resistant mat
- Tongs
Chemicals
- Copper foil, 4 cm x 4 cm
Safety and hazards
- Wear safety glasses throughout.
- Copper foil, Cu(s) – Currently not classified as hazardous, see CLEAPPS hazcard HC026. In Scotland, refer to SSERC for safety advice.
- Warn learners that there can be sharp corners on the copper.
- The copper stays hot for some time and there is a risk of burns.
Method
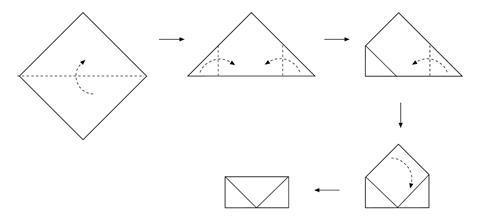
- Fold the copper foil into an envelope as shown in the diagram below.
- Wear eye protection and light the Bunsen burner.
- Hold the envelope in the tongs and heat strongly in the Bunsen flame for five minutes. You will need to have the air hole fully open.
- Place the envelope on the heat resistant mat and allow to cool. This will take a few minutes.
- Open the envelope and compare the inside to the outside surface.
Teaching notes
The outside of the envelope will react with oxygen in the air and will turn black. This can confuse learners who think that it is soot which has coated the outside of the copper. To help convince them otherwise, ensure that they use a roaring Bunsen flame and show them that a beaker (containing water) which is heated with the same flame does not get coated in black powder. Inside the envelope, the copper remains as it was at the start.
Copper, like many transition metals, only reacts slowly with oxygen in the air. When heated it forms a layer of black copper oxide on its surface:
copper + oxygen → copper oxide
2Cu(s) + O2(g) → 2CuO(s)
This experiment could be used as an illustration of the likely reactions of other transition metals with oxygen, as they all have similar properties. It could also provide a contrast to the reactions of Group 1 and 2 metals with oxygen.
Follow-up questions
Additional questions linking the practical experiment to quantitative chemistry topics can be found in the student worksheets. There are two versions of the student worksheet: scaffolded (✪) and unscaffolded (✪✪). The scaffolded sheet offers more support to allow learners to access the questions. For example, longer answer questions and equations are presented as fill-in-the-gap activities. Hints are provided after some of the questions to support learners and guide their answers.
Answers
Answers to all of the questions in both the student worksheets can be found in the teacher notes.
Downloads
Heating copper in air lesson slides
Presentation | PDF, Size 0.57 mbHeating copper in air student unscaffolded
Handout | PDF, Size 0.19 mbHeating copper in air student scaffolded
Handout | PDF, Size 0.22 mbHeating copper in air teacher notes
Handout | PDF, Size 0.25 mbHeating copper in air lesson slides
Presentation | PowerPoint, Size 1.59 mbHeating copper in air student unscaffolded
Editable handout | Word, Size 0.53 mbHeating copper in air student scaffolded
Editable handout | Word, Size 0.54 mbHeating copper in air teacher notes
Editable handout | Word, Size 0.43 mb
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. The supporting resources were updated in 2026 by Emma Bickerstaffe.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet