Try this practical to explore the reactions of ammonia with indicator solution, copper(II) sulfate solution and Nessler’s reagent
In this experiment, students observe what happens when ammonia reacts with three different test solutions using a reaction vessel in a Petri dish. They then consider how their observations might be explained.
The experiment should take about 20 minutes.
Equipment
Apparatus
- Eye protection
- Student sheet
- Clear plastic sheet (eg ohp sheet)
- Plastic Petri dish, 9 cm, base and lid
- Plastic pipette
- Scissors
Chemicals
Note
Solutions should be contained in plastic pipettes. See the accompanying guidance on apparatus and techniques for microscale chemistry, which includes instructions for preparing solutions.
- Ammonia solution, 3 mol dm–3
- Full-range indicator solution, diluted 1:1 with deionised water
- Copper(II) sulfate solution, 0.2 mol dm–3
- Nessler’s reagent (an alkaline solution of mercury iodide containing the complex ion HgI4–)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout (splash-resistant goggles to BS EN166 3).
- Wear protective gloves.
- Nessler’s reagent, K2HgI4, is EXTREMELY TOXIC by all routes and contains mercury. It is also CORROSIVE and toxic to aquatic life. Avoid contact with the skin and wash off quickly with water if this does occur.
- Ammonia solution, 3 mol dm–3 NH3(aq), is CORROSIVE – see CLEAPSS Hazcard HC006 and CLEAPSS Recipe Book RB006.
- Copper(II) sulfate solution, 0.2 mol dm–3 CuSO4(aq), causes eye damage and is toxic to aquatic life – see CLEAPSS Hazcard HC027c and CLEAPSS Recipe Book RB031.
- Some formulations of universal indicator can still be flammable at a 1:1 dilution. Keep away from sources of ignition.
Procedure
Evaporation of ammonia gas from ammonia solution:
NH3(aq) → NH3(g)
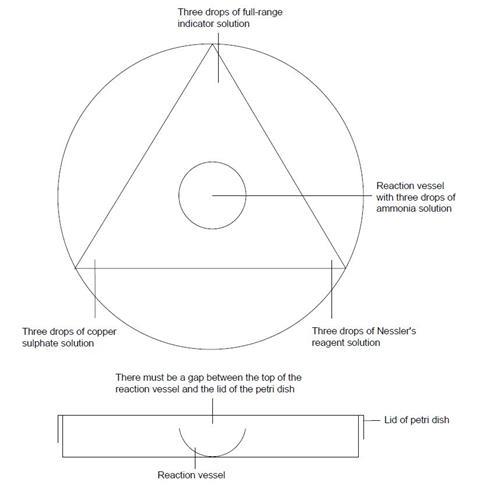
- Cover the diagram (see the student sheet) with a clear plastic sheet.
- Place the base of the Petri dish directly over the circle on the diagram. Place the reaction vessel in the centre.
- At the corners of the triangle add drops of the test solutions only as indicated below. (Take care: Nessler’s reagent is toxic – it contains mercury compounds – make sure that you do not get any on your skin. If you do, wash it off quickly with water.)
- Put three drops of ammonia solution into the reaction vessel and quickly replace the lid on the Petri dish.
- Record all your observations over the next 15 minutes.
Observations
- Full-range indicator solution turns blue-green.
- Copper(II) sulfate solution turns hazy and then develops deep blue streaks as the tetraamminocopper(II) ion is formed.
- Nessler’s reagent turns first yellow then brown. This is a very sensitive test for ammonia. The compound formed has the formula (OHg2NH2)I and consists of covalent metal–non-metal bonds which might provide an interesting point for subsequent class discussion.
Downloads
Microscale reactions of ammonia - student sheet
Editable handout | Word, Size 0.11 mbMicroscale reactions of ammonia - student sheet
Handout | PDF, Size 0.18 mbMicroscale reactions of ammonia - teacher notes
Editable handout | Word, Size 51.18 kbMicroscale reactions of ammonia - teacher notes
Handout | PDF, Size 0.15 mb
Additional information
This resource is part of our Microscale chemistry collection, which brings together smaller-scale experiments to engage your students and explore key chemical ideas. The resources originally appeared in the book Microscale chemistry: experiments in miniature, published by the Royal Society of Chemistry in 1998.
© Royal Society of Chemistry
Health and safety checked, 2018


















No comments yet