Try this demonstration to illustrate how changing chlorine concentration or temperature shifts the position of an equilibrium
In this experiment, students observe as brown liquid iodine monochloride is formed by passing chlorine gas over solid iodine. Excess chlorine converts this to yellow, solid, iodine trichloride, setting up a heterogeneous equilibrium between these three substances. Students can then see how changing the chlorine concentration or the temperature shifts the position of equilibrium in accordance with Le Chatelier’s principle.
This experiment can be used to demonstrate a reversible reaction, a heterogeneous equilibrium and the application of Le Chatelier’s principle. It must be carried out in a fume cupboard and benefits from a white background to show the colour changes to best advantage. Attention should be directed towards the U-tube to avoid distraction by the rest of the relatively complex apparatus.
The sealed U-tube can be brought out on to the bench for better visibility provided any chlorine is not allowed to escape. Although the amount of chlorine in the U-tube is small, students with breathing problems should not inhale any chlorine gas as this could trigger an asthma attack.
Provided all the apparatus is set up in a fume cupboard beforehand and the chlorine generator flushed with chlorine, the demonstration and discussion should take about ten minutes.
Equipment
Apparatus
- Eye protection (goggles – see note 2 below)
- Conical flasks, 250 cm3, x2
- Two-holed stoppers to fit flasks, x2
- U-tube with side-arms
- Stoppers to fit U-tube, x2
- Large beakers, 1–2 dm3, x2
- Tap funnel, or separating funnel with stopper
- Screw clips (Hoffmann), x2, or rubber teats (as used for glass dropping pipettes), x2 (see note 8)
- Glass tubing, bent as shown in figure
- Plastic or rubber tubing for joining glass tubes (as rubber tubing is attacked by chlorine, new tubing should be used for each demonstration)
- Access to a fume cupboard
Chemicals
- Hydrochloric acid, 5 M (IRRITANT at this concentration), about 100 cm3
- Sodium chlorate(I) solution (sodium hypochlorite) (10–14% w/v chlorine) (CORROSIVE), about 100cm3 (see note 9 below)
- Sodium hydroxide solution, at least 2 M (CORROSIVE), about 100 cm3
- Iodine (HARMFUL, DANGEROUS FOR THE ENVIRONMENT), approximately 1 g
- Crushed ice, about 250 cm3
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout – for the demonstrator this needs to be goggles because of the corrosive chemicals involved.
- Hydrochloric acid (IRRITANT at concentration used) – see CLEAPSS Hazcard HC047a and CLEAPSS Recipe Book RB043.
- Sodium chlorate(I) solution (CORROSIVE) – see CLEAPSS Hazcard HC089 and CLEAPSS Recipe Book RB081. Sometimes known as sodium hypochlorite, this is NOT the same as as sodium (or potassium) chlorate(V), NaClO3, a powerful oxidising agent which is sometimes called ‘sodium chlorate’ (without denoting the oxidation state).
- Sodium hydroxide (CORROSIVE) – see CLEAPSS Hazcard HC091a and CLEAPSS Recipe Book RB085.
- Iodine (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC054.
- Chlorine (TOXIC, IRRITANT, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC022a.
- Make sure that the pipette teats fit the side-arms of the U-tube tightly enough to prevent escape of chlorine, especially when the tube is warmed.
- Commercial chlorine-based bleach solutions (rather than household ‘bleaches’ based on peroxide, which are becoming more widely available) can be used instead of sodium chlorate(I) solution from laboratory suppliers. However, they may not be sufficiently concentrated to generate chlorine quickly enough, especially if they are old. Some commercial bleaches now also contain detergents, which foam when chlorine is generated. These should not be used.
- The contents of the chlorine generator should be disposed of by diluting with plenty of water and rinsing down the drain in a fume cupboard, once chlorine generation has ceased.
- The residue in the U-tube is corrosive. It is water-soluble and can be flushed down the sink in a fume cupboard with plenty of water. The contents of the sodium hydroxide flask can also be flushed down a sink in a fume cupboard with plenty of water.
Procedure
Before the demonstration
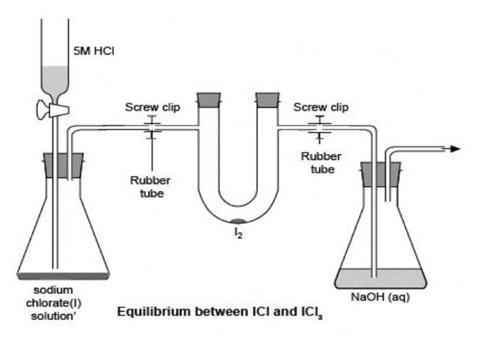
Set up the apparatus in a fume cupboard, as shown in the diagram, and place about 0.1 g of iodine in the U-tube.
The demonstration
- Generate chlorine by dripping hydrochloric acid slowly onto the sodium chlorate(I) solution. If a stoppered separating funnel is used to contain the acid, make sure the stopper is removed before opening the tap. As the chlorine gas passes over the iodine, a brown liquid (iodine monochloride, ICl) is first formed. Adjust the drop rate to maintain a steady stream of chlorine. Within a few seconds a yellow solid (iodine trichloride, ICl3) will form inside the U-tube.
- Stop adding hydrochloric acid, to reduce the flow of chlorine. Carefully release the U-tube by loosening the connections to the chlorine supply and the flask containing the sodium hydroxide. Remove the stoppers from the U-tube and invert it to allow the dense chlorine gas to flow out into the fume cupboard and be replaced by air. The yellow solid will turn back to a brown liquid. Turn the U-tube up the right way before the liquid can flow out of it.
- Replace the stoppers on the U-tube and reconnect it to the chlorine supply and the flask containing the sodium hydroxide. Restart the flow of chlorine by dripping more hydrochloric acid into the gas generator. This will result in the yellow solid reappearing in the U-tube. The demonstration can be repeated several times. Note that the first appearance of the iodine trichloride is the most clearly seen, probably because it sticks better to the clean glass.
- To demonstrate the effect of temperature on the equilibrium, refill the U-tube with chlorine again as above, then turn off the hydrochloric acid supply. Disconnect the U-tube as before and quickly seal it either by tightening the screw clips at the two positions shown in the diagram, or by pushing pipette teats onto the side-arms.
- Dip the bottom of the U-tube into a beaker of water that has just boiled. The contents of the tube will turn to the brown liquid, ICl. Now place the bottom of the U-tube into a beaker of ice water. The contents of the tube will form the yellow solid, ICl3. This cycle can be repeated many times.
Teaching notes
Students will probably be unfamiliar with interhalogen compounds formed here but should be able to see that their formation is feasible in terms of basic bonding theory. The influence of molecular mass on the intermolecular forces and hence the states of the three substances can be pointed out: it is reasonable that ICl is a liquid in view of its lower relative molecular mass than that of iodine, and similarly that ICl3 should be a solid.
The equilibrium ICl(l) + Cl2(g) ⇌ ICl3(s) is exothermic (ΔH° = -106 kJ mol–1). Lowering the chlorine concentration shifts the equilibrium towards the brown liquid, ICl, and away from the yellow solid, ICl3. Raising the chlorine concentration reverses the change. This cycle can be repeated many times.
Thus similar reversible colour and phase changes occur when the mixture is heated or cooled. An increase in temperature shifts the equilibrium to the left – an endothermic change – in accordance with Le Chatelier’s principle.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry
Equilibrium and Le Chatelier’s principle
- 1
- 2
- 3
 Currently reading
Currently readingThe effect of concentration and temperature on an equilibrium
- 4





















No comments yet