Try this game and lesson plan to reinforce learners’ understanding of bonding
This chemistry game helps learners to check and reinforce what they have learned about bonding and the links between bonding and physical properties of substances. By devising and answering questions in a fun, competitive environment their understanding of bonding and some key substances is reinforced.
-

Download this
Bonding bingo, for age range 14–16
Downloads include lesson powerpoint and teaching sequence; bingo and substance cards; teacher guidance and learner instructions.
View and download more Lesson plans
This activity would be particularly useful for checking learning towards the end of teaching this topic or when revising for an external assessment.
Learning objectives
- Describe the structure and bonding of ionic, simple covalent, giant covalent and metallic structures.
- Explain how the physical properties associated with these substances relate to their structure and bonding.
Teaching sequence
Introduction (slides 3–6)
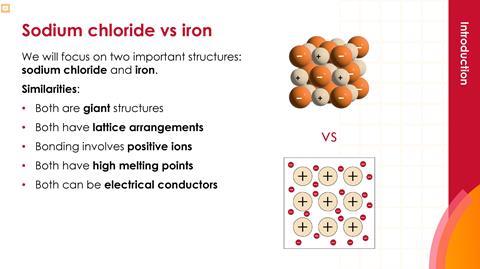
- Introduce the two key substances, sodium chloride and iron. Distribute mini whiteboards.
- Ask learners to write down on their mini whiteboards one similarity between the structure or properties of sodium chloride and iron.
- Ask learners to compare their answer to their neighbours’ responses.
- Come together as a group and review the key similarities.
- Repeat the task, but this time ask learners to identify one difference between the structure or properties of sodium chloride and iron.
Bonding bingo: (slides 7–9)
- Group learners into teams of two.
- Explain the foal of the task: to identify the substances that opposing teams have on their card, eventually crossing off all nine substances from their bingo grid.
- Emphasise that they need to use the 10 minutes before the game starts to devise and write down questions: which have a yes or no answer, that will help to find out which substance is being described.
- Give each team a ‘Bonding bingo student sheet’ to write down their questions. For extra support, move around the teams as they devise their questions and offer prompts as needed.
- Display the nine possible substances to guide learner thinking.
- Give each team one ‘Bingo card’ and one ‘Substance name card’ (several pairs may have the same name).
- Explain the rules of ’Bonding bingo’:
- A team sits with another team.
- They toss a coin to decide which team asks questions first.
- The questioners have to find out what substance is on the other team’s substance name card by asking questions from their list.
- The other team must answer either yes or no.
- They will only have one guess to correctly identify the substance.
- If it is correct, the other team initials the substance square on the questioner’s Bingo card .
- The roles are now reversed with the opposite team asking and answering questions.
- The process is repeated with the other team asking the questions.
- When completed, the two teams each go and join up with a different team, they go around the room until one team correctly identifies all nine substances and house ‘bingo’!
Reflections (slide 10)
Bring students together in a plenary. Ask:
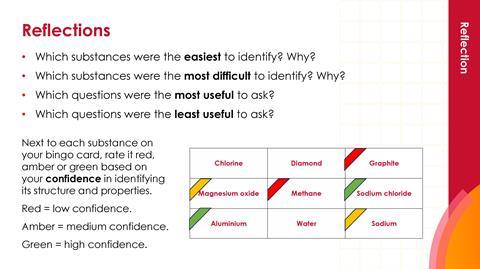
- Which substances did you find easy to identify?
- Which substances did you find more difficult to identify?
- Which questions helped you the most and which were less useful?
Provide suitable small prizes for the winners of Bonding bingo! Encourage learners to reflect on this activity by rating their confidence (red, amber or green) to identify the structure and properties of each substance.
Commentary
As learners devise and discuss their questions they reassess what they understand about the structure and properties of the substances. During the game this is constantly tested by the other member of their pair and by the other teams, as they seek or give answers to the yes/no questions.
The plenary and personal reflection will give you an indication of the overall level of understanding and allow learners to think about how confident they are with this topic.
Scaffolding
- For the introduction task, specify that learners identify similarities and differences between the bonding, then the structures, then the properties. This will encourage learners to deepen their understanding of each concept.
- For the Bonding bingo, provide learners with example questions as a prompt.
- Additionally, come together as a group after the 10 minutes’ preparation time to share suggestions or questions.
- Consider breaking this task into two; stop when the first group achieve ‘three in a row’, to discuss which questions work well. Then ask learners to compete again until they cross off all nine.
Language support for structure and bonding
Find key terms support resources, including an accessible glossary, Frayer models and unscrambling definitions worksheet for this topic. A reading comprehension, structured talk activity and structure strips are also available.
Get literacy support
Challenge opportunities
- Ask learners to note down how many questions they asked before identifying the substance. Reward teams who asked the least number of questions.
- Limit the number of questions learners are allowed to ask before making a guess e.g. ‘You may only ask five questions.’
- Limit the amount of time learners have for asking questions, e.g. to two minutes, before a guess must be made.
- Specify how many structure, bonding and property questions can be asked e.g. ‘You can only ask two questions about the chemical bonding on the substance’.
- Change the substances, e.g. incorporate graphene, which requires learners to understand the difference between graphene and graphite.
Answers
Similarities and differences
Some observations learners might make about the similarities and differences between sodium chloride and iron:
Similarities
- Both are giant structures.
- Both have lattice arrangements.
- Bonding involves positive ions.
- Both have high melting points.
- Both can conduct electricity.
Differences
- Only iron has delocalised electrons.
- Sodium chloride’s bonding involves positive and negative ions; iron’s bonding only involves positive ions.
- Only sodium chloride dissolves in water.
- Iron is a shiny grey solid at room temperature; sodium chloride is a white crystalline solid.
- Iron conducts electricity as a solid, sodium chloride doesn’t.
Possible learner questions
- Does the substance have a giant lattice?
- Does the substance contain ions?
- Does the substance contain simple molecules?
- Does the substance have a high melting point?
- Is the substance a gas at room temperature?
- Does the substance conduct electricity?
- Does the substance dissolve in water?
- Is there more than one delocalised electron per ion?
Downloads
Bonding bingo lesson slides
Presentation | PDF, Size 1.03 mbBonding Bingo student worksheet
Handout | PDF, Size 0.18 mbBonding bingo teacher notes
Handout | PDF, Size 0.33 mbBonding bingo lesson slides
Presentation | PowerPoint, Size 21.4 mbBonding bingo student worksheet
Editable handout | Word, Size 0.47 mbBonding bingo teacher notes
Editable handout | Word, Size 0.61 mb
Additional information
This lesson plan was originally part of the Assessment for Learning website, published in 2008. It was updated in 2011 by Louise Glynn.
Assessment for Learning is an effective way of actively involving students in their learning. Each session plan comes with suggestions about how to organise activities and worksheets that may be used with students.


















No comments yet