Practise writing formulas for ionic compounds and revise common positive and negative ions using this lesson plan with activities
This resource is from the Assessment for learning series, which contains lesson plans and associated resources to actively involve students in their learning.
-

Download this
Writing formulas for ionic compounds, for age range 14–16
Downloads include lesson slides and teaching sequence; interactive ion cards to print and cut out; standard and challenge student worksheets; teacher guidance and answers.
View and download more Lesson plans
In this activity, ion formula cards help learners check, consolidate and demonstrate their ability to write correct formulas for ionic compounds. Learners work cooperatively in groups to demonstrate their mastery of the ideas. Once one group has had their work checked they will peer assess other groups so learning cascades around the classroom.
Learning objectives
- Recall the names and formulas of common positive ions and negative ions.
- Write formulas of ionic compounds.
Sequence of activities
Introduction (slides 2–4)
- Display the images of magnesium nitride and magnesium nitrate.
- Ask learners the differences between the compounds. Prompt them to go beyond the images, to think about what the names mean. Encourage learners to recall other examples of ‘-ides’ and ’-ates’ e.g. sulfide, slafate. Why doesn’t oxygen have ‘oxate’?
- Discuss the difference between the two formulas. Learners may discuss the different elements and how many there are. Guide learners to think about the ions - both contain Mg2+ ions, but what is different about the negative ion? How does this impact the overall ionc formula?

Activity 1: ion formulas (slides 6–7)
- Organise learners into groups of four and ask them to complete Activity 1 on their student sheets by adding the names of the ions listed. You can give learners the standard student sheet (MS Word | PDF) or the challenge sheet (MS Word | PDF).
- When one group believes it has completed this task successfully, check their work. If successful, designate them an ’expert group’. Authorise these group members to check the work of other groups.
- Allow the checking to cascade.
- Ask learners to self-assess their work using the answers displayed on the screen.
Combining formulas (slides 8–13)
Give each learner a mini-whiteboard. For each of the three examples, ask learners to:
- Write the formulas of the positive and negative ions.
- Write the formulas of the ionic compounds.
Use the shapes to help explain to learners. Emphasise the use of subscript numbers and when brackets are needed.
Activity 2: formulas for ionic compounds (slides 14–15)
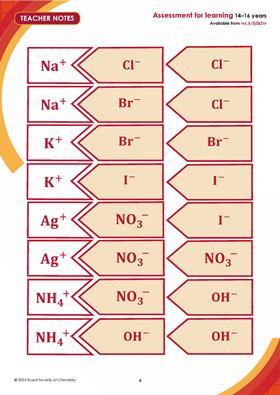
- Provide learners with the Ion formula cards (at the end of the teacher guidance, MS Word | PDF)
Ask them to: arrange the cards to form the formulas of the ionic compounds on their sheet then write down the formulas of the ionic compounds. - As before, when one group has finished, check their work using random questions. If five questions are correctly answered, designate them an ‘expert group’ Authorise these group members to check the work of other groups and to designate then as an ‘expert group’.
- Allow the checking to cascade.
- Ask learners to self-asses their work using the answers displayed on the screen.
Note: the student sheet includes the ionic compound sodium hydrogen carbonate, which does not appear on some specifications. Learners should be able to complete the activity by applying the same principles as they have to the other samples but you can choose to replace the ionic compound with an alternative depending on the exam board and specification e.g. ammonium carbonate.
Review (slide 16)
With the whole class, ask learners to:
- Write down the ions contained in magnesium nitride and then magnesium nitrate (review from introduction).
- Compare this with what was discussed at the start of the lesson.
- Ask learners to write a brief explanation of the differences between the formulas of magnesium nitride and magnesium nitrate. Encourage learners to draw diagrams, similar to those used in Activity 2.
- Get learners who completed the extension in the starter to do the same for any other ‘ides’ and ‘ates’ they listed.
Commentary
Use the ‘nitrate vs nitride’ introduction to show learners how compounds are different based on their names and formulas, and the importance of ‘ide’ vs ‘ate’ in chemical formulas.
In the two main activities get learners to support and assess each other, while using visual prompts such as ion cards to support their understanding.
By using the task of personal evaluation, asking learners to use the mini whiteboards, as well as returning to the introduction task at the end of the lesson, you will promote learners’ confidence that they can improve.
Scaffolding
Provide learners with the ionic formula of magnesium nitride and magnesium nitrate (slide 3) during the introduction to encourage them to spot the differences in the elements they contain.
For Activity 1, you can give learners the standard sheet (MS Word | PDF), which gives learners the ion formulas, requiring only the names to be completed.
Alternatively, give them the challenge sheet (MS Word | PDF), which requires learners to complete a mixture of ion names and their formulas.
For Activity 2, you can decide whether learners need to use the ion cards, based on their understanding of the three whole-class examples.
You can ask learners to create further ionic formulas using the cards as an extension opportunity.
Answers
The formulas featured in the resource are:
- Magnesium carbonate – MgCO3
- Silver nitrate – AgNO3
- Calcium bromide – CaBr2
- Copper hydroxide – Cu(OH)2
- Iron(II) nitrate – Fe(NO3)2
- Iron(III) iodide – FeI3
- Lead sulfate – PbSO4
- Zinc nitrate – Zn(NO3)2
- Potassium sulfate – K2SO4
- Magnesium sulfide – MgS
- Aluminium hydroxide – Al(OH)3
- Ammonium chloride – NH4Cl
- Sodium hydrogen carbonate – NaHCO3
- Iron(III) carbonate – Fe2(CO3)3
Downloads
Writing ionic formulas lesson slides
Handout | PDF, Size 0.81 mbWriting formulas standard student sheet
Handout | PDF, Size 0.21 mbWriting formulas student challenge sheet
Handout | PDF, Size 0.21 mbWriting formulas teacher notes
Handout | PDF, Size 0.73 mbWriting ionic formulas lesson slides
Editable handout | PowerPoint, Size 31.23 mbWriting formulas standard student sheet
Editable handout | Word, Size 0.45 mbWriting formulas student challenge sheet
Editable handout | Word, Size 0.46 mbWriting formulas teacher notes
Editable handout | Word, Size 0.79 mb
Additional information
This lesson plan was originally part of the Assessment for Learning website, published in 2008. It was updated in 2011 by Louise Glynn and Kirsty Patterson.
Assessment for Learning is an effective way of actively involving students in their learning. Each session plan comes with suggestions about how to organise activities and worksheets that may be used with students.




























No comments yet