Discover signs of an oxidisation reaction, and form cyclohexadnone, with this quick practical
This is a quick microscale experiment to illustrate that alcohols like cyclohexanol react with acidified dichromate(VI). No attempt is made to identify the product. The experiment takes about 15 minutes.
Equipment
Apparatus
- Eye protection (goggles)
- Petri dish, 5.5 cm size, plastic
- Dropping pipettes x2
- White paper
Chemicals
- Cyclohexanol (HARMFUL)
- Potassium dichromate(VI) (OXIDISING, VERY TOXIC, DANGEROUS FOR THE ENVIRONMENT), 2 g (note 4)
- Concentrated sulfuric acid (CORROSIVE)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection (goggles) throughout.
- Cyclohexanol, C6H11OH(s) or (l) (HARMFUL) – see CLEAPSS Hazcard HC084C. Cyclohexanol has a melting point of 20–22 °C (about room temperature). If this experiment is done on a warm day the cyclohexanol is liquid and therefore easier to sample. In cold laboratories, warm the cyclohexanol first.
- Potassium dichromate(VI), K2Cr2O7(s), (OXIDISING, VERY TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC078c. To make up the the acidified dichromate(VI) solution: dissolve 2 g of potassium dichromate(VI) in 80 cm3 of deionised or distilled water and slowly add 10 cm3 of concentrated sulfuric acid to the solution, with cooling. Label the solution TOXIC and CORROSIVE.
- Concentrated sulfuric acid, H2SO4(l), (CORROSIVE) – see CLEAPSS Hazcard HC098a.
Procedure
- Place a Petri dish on a piece of white paper.
- Collect 1 cm3 of acidified potassium dichromate(VI) solution in a dropping pipette.
- Carefully, use the pipette to place ten drops of potassium dichromate(VI) in the centre of the dish.
- Use another pipette to place one drop of cyclohexanol into the pool of dichromate(VI) solution.
- Observe the pool over the next few minutes. Look for signs of reaction and of new substances being formed.
Teaching notes
A great deal of surface activity is observed.
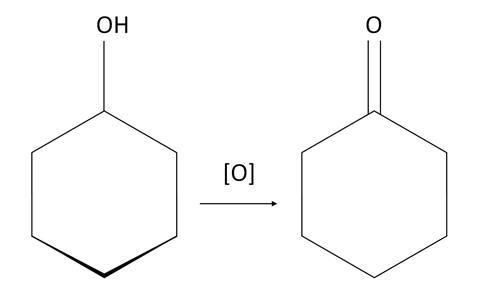
This is due to the differences in physical properties such as surface tension and viscosity of the product (cyclohexanone) compared to the reactant (cyclohexanol). A green colour, due to Cr3+(aq), will also be seen.
The reaction can be represented like this:
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet