Quiz your students on their mathematical competencies
This Starter for ten covers the following topics:
- rearranging equations
- BODMAS (order of operations)
- quantity calculus (unit determination)
- expressing large and small numbers
- significant figures, decimal places and rounding
- unit conversions – length, mass, time and volume,
- moles and masses
- moles and concentration.
Example questions
The order of operations for a calculation is very important. If operations are carried out in the wrong order then this could lead to the wrong answer. Most modern calculators will anticipate BODMAS issues when operations are entered but human beings can override the calculator’s instincts.
1. Do the following calculations in your head
- 3 + 5 × 5 =
- 6 × 6 + 4 =
- 20 – 6 × 2 =
- 48 – 12 ÷ 4 =
- 4 + 4 ÷ 2 =
- 100 – (20 × 3) =
(6 marks)
The molecular formula of glucose is C6H12O6. Three students entered the following into their calculators to calculate the relative formula mass of glucose. Repeat their calculations as shown.
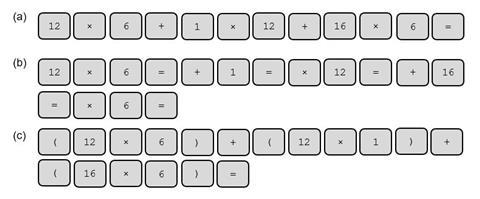
Write a sentence summing up why the answers differ.
(4 marks)
Large and small numbers are often expressed using powers of ten to show their magnitude. This saves us from writing lots of zeros, expresses the numbers more concisely and helps us to compare them.
In standard form a number is expressed as;
a × 10n
where a is a number between 1 and 10 and n is an integer.
Eg, 160 000 would be expressed as 1.6 × 105
Sometimes scientists want to express numbers using the same power of ten. This is especially useful when putting results onto a graph axis. This isn’t true standard form as the number could be smaller than 1 or larger than 10. This is more correctly called scientific form.
Eg, 0.9 × 10–2, 2.6 × 10–2, 25.1 × 10–2 and 101.6 × 10–2 are all in the same scientific form.
Express the following numbers using standard form.
- 1 060 000
- 0.001 06
- 222.2
Notes
A full version of the question and answer sheets are available in the ‘Downloads’ section below, an editable version of both of these sheets is also available.
Downloads
Basic mathematical competencies – answer sheet
PDF, Size 0.32 mbBasic mathematical competencies – answer sheet
Word, Size 0.13 mbBasic mathematical competencies – question sheet
PDF, Size 0.66 mbBasic mathematical competencies – question sheet
Word, Size 0.38 mb
Additional information
Developed by Dr Kristy Turner, RSC School Teacher Fellow 2011–2012 at the University of Manchester, and Dr Catherine Smith, RSC School Teacher Fellow 2011–2012 at the University of Leicester.
1. The amount of substance in moles (n) in a solution can be calculated when the concentration given in mol/dm3 (c) and volume (v) in cm3 are known by using the equation:
![]()
a. (1 mark)
b. (1 mark)
2.
![]()
a.
3. However, the SI unit for density is kg/m3.
b. 3 when the mass (m) of the substance is given in g and the volume (v) of the substance is given in cm3.
3.
![]()
a.
![]()
b.
4.
![]()
Starters for 10: Transition skills (16–18)

This important chapter in our Starters for ten series covers: chemistry competencies, mathematical competencies, and practical competencies. Helping embed the skills required for the transition to advanced level courses.
- 1
- 2
 Currently
reading
Currently
reading
Basic mathematical competencies
- 4

























No comments yet