Try this practical to remove objectionable tastes and odours from water using carbon in the form of activated charcoal
Carbon that has undergone special treatment (sometimes called activated charcoal) has useful absorption properties, capable of removing coloured and volatile material from mixtures. In this experiment, students use activated charcoal to tackle unwanted colours and odours in water, created by the addition of ink or food colouring and malt vinegar.
The experiment reflects the wide application of activated charcoal in organic chemistry and industry, including its use for the removal of objectionable tastes and odours from drinking water or medicines.
The practical is conveniently carried by groups of two or three and will take about 45 minutes.
Equipment
Apparatus
- Eye protection
- Beaker, 100 cm3
- Filter funnel
- Filter paper
- Test tubes, x2
- Test tube rack
- Spatula
Chemicals
- Activated charcoal, ten spatulas full
- Ink or food colouring, one drop (see note 5 below)
- Malt vinegar, 100 cm3 (see note 6)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Activated charcoal, C(s) – see CLEAPSS Hazcard HC021.
- Malt vinegar – see CLEAPSS Hazcard HC038a.
- Fountain pen ink (‘washable’ variety) is the best type of ink to use. A dilute solution of potassium permanganate(VII) could be used instead of ink or food flavouring.
- Juice from sauerkraut or pickles could be used instead of malt vinegar.
Procedure
- Fold a piece of filter paper, place it in a funnel, and put the stem of the funnel into a test tube in a test tube rack.
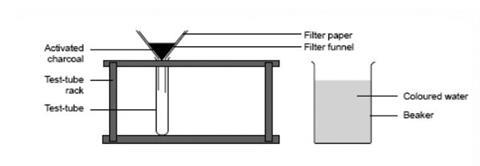
- Add about five spatulas of activated charcoal to the funnel.
- Add one drop of ink or food colouring to 100 cm3 of water in a beaker.
- Carefully pour some of the coloured water on to the charcoal in the filter paper. Note whether the drops of liquid in the test tube have lost the original colour.
- Repeat the activity with another test tube, this time pour 100 cm3 of malt vinegar through the charcoal. Note whether the filtered liquid has lost some of its original strong smell.
Teaching notes
Students need to be warned that activated charcoal powder is extremely messy and difficult to remove from clothing.
The vinegar still smells after filtration, but noticeably less so.
Background theory
Heating wood to a very high temperature in the absence of air makes charcoal. When it is heated to an even higher temperature – about 930 °C – impurities are driven from its surface and it becomes ‘activated charcoal’. This activated charcoal can remove impurities in either the gaseous or liquid state from many solutions. It does so by the process of adsorption, by attracting these molecules to the surface of the charcoal.
Adsorption by charcoal is also used to remove unburned hydrocarbons from car exhausts, harmful gases from the air, and unwanted colours from certain products.
Students may find the difference between adsorption and absorption confusing. Adsorption is a process in which a gas, liquid, or a dissolved substance is gathered on the surface of another substance – eg charcoal. Absorption is a process in which a liquid is soaked up, as with blotting paper. It is taken in completely and mixes with the absorbing material – eg absorbent cotton.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet