Research suggests that learners commonly focus on covalent and ionic bonding, and often fail to spot, or may down-play the importance of, other types of bonding
A variety of types of diagram are used in this activity, to help learners be able to interpret and use various ways of representing chemical species.
How to use this resource
Use this resource to quickly audit learners’ awareness of different bond types. (Use the Interactions resource to explore post-16 learners’ more detailed understanding of the same topic.)
Point out to learners that some of the diagrams refer to individual atoms or molecules, while others show some of the particles in named substances and they should therefore pay close attention to the labels under the figures.
- When to use? Use to develop or assess 16–18 learners who have completed studying bonding at post-16 level.
- Group size? Suitable for independent work in class to diagnose learners’ misconceptions.
- Topics assessed? Chemical bonding (including: ionic, covalent, metallic, polar, hydrogen, dipole-dipole, van der Waals, solvation, dative, double, delocalised).
- How long? 10–15 minutes
Activity
This exercise comprises of a set of diagrams showing a range of chemical species and systems. For each diagram: either write the name or names of the type or types of bonding present, or write none (if there is no chemical bonding) or do not know if you are unsure.
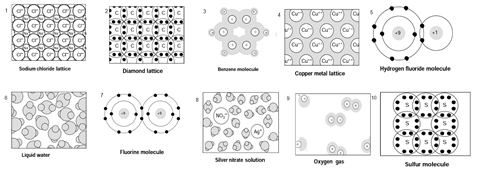
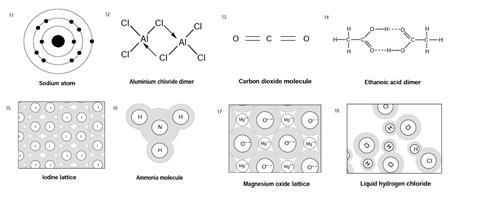
Rationale
Research suggests that learners focus on covalent and ionic bonding, and can either miss, or down-play the importance of, other types of bonding. Read a discussion of learners’ ideas about atomic structure and other chemical structures.
A variety of types of diagram are used in this resource, as it is important for learners to be able to interpret and use various ways of representing chemical species (read more about learners’ beliefs in alternative ideas).
-

Scaffolding
An alternative version of this resource has been adapted for learners aged 14–16 years.
The 14-16 version includes lesson slides, two levels of worksheet (one with fewer diagrams selected for their familiarity to 14–16 learners) and an acknowledgement of the limited range of bond types met at the 14–16 level.
Scaffolding
An alternative version of this resource has been adapted for learners aged 14–16 years.
The 14–16 version includes lesson slides, two levels of worksheet (one with fewer diagrams selected for their familiarity to 14–16 learners) and an acknowledgement of the limited range of bond types met at the 14–16 level.
Answers
The following answers are suitable for students who have studied bonding at post-16 level. Where the Spot the bonding probe is used with students at an earlier stage, then they should not be expected to provide the full range of responses.
- Sodium chloride lattice: ionic
- Diamond lattice: covalent
- Benzene molecule: covalent, delocalised
- Copper lattice: metallic
- Hydrogen fluoride molecule: covalent, polar
- Liquid water: covalent, polar; hydrogen, covalent, van der Waals forces, dipole-dipole forces
- Fluorine molecule: covalent
- Silver nitrate solution: covalent, polar; hydrogen, dipole-dipole, van der Waals forces, solvent-solute interactions
- Oxygen gas: covalent (double/sigma + pi), van der Waals forces
- Sulfur molecule: covalent
- Sodium atom: no chemical bonding (although intra-atomic forces of similar nature)
- Aluminium chloride dimer: polar, including dative (coordinate) covalent
- Carbon dioxide molecule: covalent, polar (double/sigma + pi)
- Ethanoic acid dimer: covalent, polar, hydrogen
- Iodine lattice: covalent, van der Waals forces
- Ammonia molecule: covalent, polar
- Magnesium oxide lattice: ionic
- Liquid hydrogen chloride: covalent, polar, van der Waals forces
Notes
- Where a bond has significant polarity, it could be described as polar rather than covalent (or polar covalent).
- The term van der Waals forces has been used for induced dipole-dipole forces.
- Students may forget to mention van der Waals forces in cases where they recognise hydrogen-bonds are present (i.e. items 6, 8 and 18).
- The presence of some covalent character in the magnesium oxide lattice may be spotted by some students.
Downloads
Spot the bonding student sheet 16–18
Handout | PDF, Size 0.59 mbSpot the bonding teacher notes 16–18
Handout | PDF, Size 0.23 mbSpot the bonding student sheet 16–18
Editable handout | Word, Size 1.06 mbSpot the bonding teacher notes 16–18
Editable handout | Word, Size 0.6 mb
Websites
Additional information
These resources have been taken from the book Chemical Misconceptions: Prevention, Diagnosis and Cure: Theoretical Background, Volume 2, by Keith Taber.
The resource was adapted and updated in 2025 by Kirsty Patterson.

Chemical misconceptions

Discover classroom strategies and activities to tackle common misconceptions among students in chemistry, and explore the theory behind different approaches.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
 Currently
reading
Currently
reading
Spot the bonding | 16–18 years
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36






























































































No comments yet