Use this practical to introduce learners to physical and chemical changes and the safe use of Bunsen burners
-

Download this
A ready-to-go practical lesson with classroom slides, scaffolded and unscaffolded student worksheets and teacher guidance, including full technical notes and answers to all questions.
Discover more resources from the Nuffield practical collection
In this simple experiment, learners use a Bunsen burner and water bath to investigate the different effects of heat on chocolate and egg white. The practical provides a clear introduction to physical and chemical changes, and can be used to ensure students learn how to use Bunsen burners safely.
This straightforward, if somewhat messy, experiment should take no more than 30 minutes.
Learning objectives
- Safely heat egg white and chocolate using a Bunsen bruner and record observations.
- Describe and explain observations from a chemical reaction.
- Categorise statements as relating to chemical or physical changes.
- Distinguish wheter a change is chemical or physical from given observations.
Scaffolding
There are two versions of the student worksheets: scaffolded and unscaffolded. The scaffolded sheet offers more support to allow learner to access the questions. For example, answers require learners to choose from a series of pre-populated answers or to fill in gaps for longer answer questions. Hints are found after some of the questions to support learners further and guide their answers.
Integrated instructions are available in the lesson presentation slides.
Technical notes
Read our standard health and safety guidance and carry out a risk assessment before running any live practical.
Equipment
Apparatus
- Safety glasses
- Test tubes, x 2
- Beaker, 250 cm3
- Bunsen burner
- Heat resistant mat
- Tripod
- Gauze
- Test tube rack
- Test tube holder
Chemicals
- Chocolate, a few grams
- Egg albumen (egg white), about 4 cm3
Health, safety and technical notes
- Wear safety glasses throughout.
- Do not sit down while heating the beaker or handling the hot test tubes.
- Do not taste foods in a laboratory. The food or the apparatus may be contaminated. This ‘no tasting’ rule should be strictly enforced.
- Cooking chocolate is the best type of chocolate for this experiment (other types melt too slowly). Grate the chocolate and pre-load it into a test tube for each working group, sufficient to give about a 2 cm depth of molten chocolate when heated – this may require trials to establish the correct amount of grated chocolate.
- In the UK use eggs with the ‘lion brand’ stamp as these should be salmonella free. Separate the egg whites from the yolks of sufficient eggs to provide enough for about 4 cm3 of egg white for each working group. Pre-load the egg with into a test tube for each working group.
- At the end of the lesson, ask learners to hand back their test tubes with the contents still inside, as recovery and cleaning may cause less mess than leaving it to the learners. The chocolate is best removed by re-melting and pouring out of the tubes.
Method
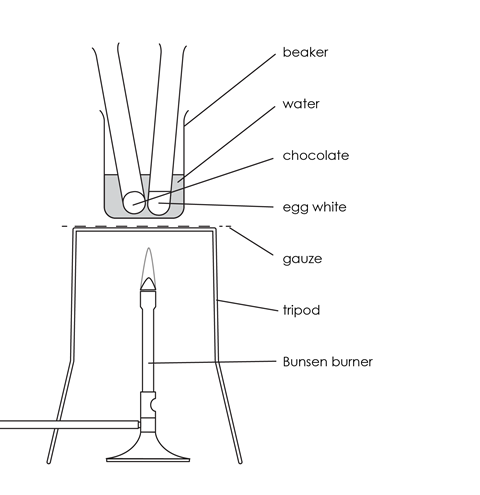
Source: Royal Society of Chemistry
How to set up the water bath for heating chocolate and egg white over a Bunsen burner
- Add cold water to the beaker until it is about one-third full, and place it on the tripod and gauze.
- Place a test tube with egg white and a test tube with chocolate in the beaker.
- Heat the beaker of water with the test tubes carefully until the water in the beaker boils. Allow the water to boil gently for about 5 minutes.
- Watch what happens to the egg white and the chocolate in the tubes while they are being heated.
- Turn off the Bunsen burner and use the test tube holder to transfer the tubes to the rack to cool.
- Watch what happens to the egg white and the chocolate in the tubes as they cool.
Teaching notes
This experiment is appropriate for classes at an early stage in their science education, so learners are likely to be fairly inexperienced in the safe and skillful use of the Bunsen burner. This is a good opportunity to develop their ability to use the Bunsen burner and emphasise safety points, such as standing up when doing experiments that involve heating.
The main purpose of the experiment is to introduce physical and chemical change, and the associated ideas of reversible and non-reversible changes. The chemical change in the egg white should take no more than 5 minutes once the water is boiling, and grated cooking chocolate should melt in about the same time. Many learners will have met this in primary school.
On cooling, the chocolate will of course solidify to a solid mass, and learners may be distracted by the change of form from the grated material at the start. If so, the technician could be asked to pre-melt the chocolate in the tubes and allow it to solidify again before the lesson, but note that it will then take longer for the chocolate to melt in the experiment.
Follow-up questions
Answers to the follow-up questions in the student worksheets and on the lesson slides can be found in the teacher notes.
Further information
The Exploratorium provides a discussion of the nature of the chemical changes involved in cooking eggs, suitable for teacher background information.
Downloads
Heating chocolate and egg unscaffolded student sheet
Handout | PDF, Size 0.28 mbHeating chocolate and egg scaffolded student sheet
Handout | PDF, Size 0.29 mbHeating chocolate and egg lesson slides
Presentation | PDF, Size 1.16 mbHeating chocolate and egg teacher notes
PDF, Size 0.25 mbHeating chocolate and egg unscaffolded student sheet
Editable handout | Word, Size 0.63 mbHeating chocolate and egg scaffolded student sheet
Editable handout | Word, Size 0.63 mbHeating chocolate and egg teacher notes
Editable handout | Word, Size 0.48 mbHeating chocolate and egg lesson slides
Presentation | PowerPoint, Size 11.21 mb
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry
The resource was updated in 2026 with additional follow-up questions, teacher notes and clasroom slides added by Emma Bickerstaffe.


















No comments yet