Quiz your students on their redox knowledge
The topics covered in this Starter for ten activity are: equipment, treatment of errors, titrations, observation exercises, inferences and managing risk.
Example questions
You have just started in a new research lab and have the following equipment available to you. Choose which piece(s) of equipment you would use for each of the following tasks and name each piece you use. You can assume you have plenty of bungs, tubing and adaptors plus access to a safe source of heat;
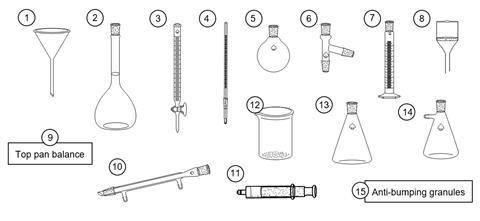
- To measure the volume of carbon dioxide produced by the following reaction;
ethanoic acid + sodium hydrogen carbonate → sodium ethanoate + water + carbon dioxide
- To obtain pure water from a sample of salt water
- To oxidise a sample of propan-1-ol to propanoic acid by heating with an excess of an acidified aqueous solution of sodium dichromate under reflux conditions
- To make up an accurate 0.05 mol dm–3 solution of sodium hydroxide
- To recrystallise a sample of paracetamol contaminated with an insoluble solid impurity and collect the solid product
A student submitted the following report on his recent experiment to determine the concentration of a solution of hydrogen peroxide;
Method
10 cm3 of the hydrogen peroxide was measured using a 100 cm3 measuring cylinder and placed in a 100 cm3 conical flask.
A bung and tubing was attached and the other end connected to a 100 cm3 gas syringe. 0.2 g of the MnO2 catalyst was weighed out.
The catalyst was added to the hydrogen peroxide solution and the bung quickly replaced.
The total volume of oxygen produced was measured.
Results
| Experiment | 1 | 2 | 3 |
|---|---|---|---|
|
Mass of MnO2 / g |
0.21 |
0.24 |
0.20 |
|
Volume of gas produced / cm3 |
15 |
13 |
13 |
Analysis
The equation for the reaction is: 2 H2O2 → 2 H2O + O2
Average volume of gas produced = 13.7 cm3
Assuming room temperature and pressure, no. of moles in 13.7 cm3 of gas = 13.7 cm3 ¸ 24,000 cm3 mol–1 = 5.694 x 10-4 moles
2 moles of H2O2 produce 1 mole of O2 so the no. of moles of H2O2 in 10 cm3 = 1.139 x 10–3 moles
\concentration of H2O2 solution = 1.139 x 10-3 mol ¸ 0.010 dm3 = 0.1139 mol dm–3
1. The accuracy of each piece of equipment used is shown below.
(a) Using the individual equipment errors listed below, calculate the total percentage error for the reaction.
| 100 cm3 measuring cylinder ± 0.5 cm3 | 100 cm3 gas syringe ± 0.5 cm3 |
|---|---|
|
100 cm3 conical flask ± 5 cm3 |
Top pan balance ± 0.005 g
|
(b) Suggest how this percentage error could have been reduced using the same equipment.
2. The teacher wants to give the student some feedback on how he could have improved the accuracy of his experiment. Suggest two pieces of feedback she could give him.
Comment on the student’s use of significant figures in his analysis. From your comments, correct the student’s final concentration of the hydrogen peroxide.
Notes
A full version of this question and answer sheet is available from the ‘Downloads’ section below. An editable version is also available.
Downloads
Experimental skills
Word, Size 0.43 mbExperimental skills
PDF, Size 0.47 mb
Starters for 10: Advanced level 1 (16–18)

This chapter in our Starters for ten series covers quantitative chemistry, atomic structure, bonding, trends in the periodic table, organic chemistry, thermodynamics, kinetics, equilibria, redox, analysis and experimental skills.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
 Currently
reading
Currently
reading
Experimental skills









































No comments yet