Quiz your students on types of bonding
The topics covered in this Starter for ten activity are: covalent dot and cross; ionic dot and cross; which typr of chemical bond; co-ordinate bonding; electronegativity and polarity; intermolecular forces; shapes of molecules; and properties of bonding.
Example questions
Draw dot and cross diagrams to illustrate the bonding in the following covalent compounds. If you wish you need only draw the outer shell electrons;
(2 marks for each correct diagram)
- Water, H2O
- Carbon dioxide, CO2
- Ethyne, C2H2
- Phosphoryl chloride, POCl3
- Sulfuric acid, H2SO4
Draw dot and cross diagrams to illustrate the bonding in the following ionic compounds.
(2 marks for each correct diagram)
- Lithium fluoride, LiF
- Magnesium chloride, MgCl2
- Magnesium oxide, MgO
- Lithium hydroxide, LiOH
- Sodium cyanide, NaCN
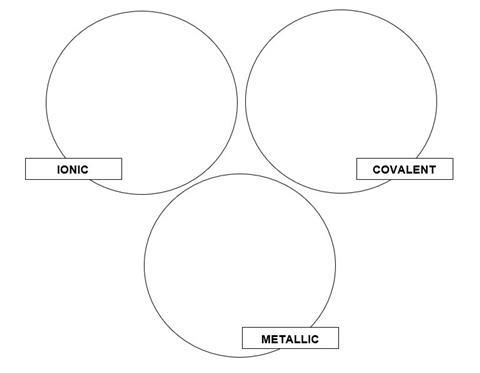
There are three types of strong chemical bonds; ionic, covalent and metallic.
1. Sort the compounds below into groups within the circles below according to their chemical bonding;
|
sodium chloride, NaCl |
magnesium, Mg |
magnesium oxide, MgO |
|
methane, CH4 |
oxygen, O2 |
barium iodide, BaI2 |
|
aluminium, Al |
ammonia, NH3 |
caesium, Cs |
Notes
A full version of this resource, including questions and answers, is available from the ‘Downloads’ section below. An editable version is also available.
Downloads
Bonding – editable
Editable handout | Word, Size 1.06 mbBonding
Handout | PDF, Size 1.06 mb
Starters for 10: Advanced level 1 (16–18)

This chapter in our Starters for ten series covers quantitative chemistry, atomic structure, bonding, trends in the periodic table, organic chemistry, thermodynamics, kinetics, equilibria, redox, analysis and experimental skills.
- 1
- 2
- 3
 Currently
reading
Currently
reading
Bonding
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12









































2 readers' comments