Investigate hydrogels as polymeric smart materials in this series of activities using nappies and cheap hair gel
In this pair of experiments, students explore the properties of hydrogels used in commonly available products – in this case, disposable nappies and cheap hair gel. The practical work is fun to do, and the results are sudden and dramatic.
To do all the practical work takes about 30 minutes. The hair gel experiment is a good quick introduction to hydrogels, while the nappy experiment is more detailed.
If time is available, it is worth considering combining this practical with this experiment exploring hydrogels in the context of plant water storage crystals.
It is a good idea to ask students to make detailed observations of each part of the experiment.
Equipment
Apparatus
- Eye protection
- Plastic gloves for those with sensitive skin
Hair gel experiment
- Hair gel
- Petri dish or lid
- Teaspoon or similar – an ordinary spatula is a bit small
Disposable nappies experiment
- Scissors
- Large ice cream tub or similar container (see note 6 below)
- Dessert spoon or similar measure
- Stirring rod
- Large beaker or plastic tub to hold at least 600 cm3
Chemicals
- Disposable nappy, x1
- Distilled water, about 500 cm3 per group
- Access to hair gel
- Access to sodium chloride (table salt)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Hair gel – the cheaper and nastier the better. Allow about one large teaspoonful per group.
- Disposable nappy – Pampers Baby Dry® nappies work well, but any ultra-absorbent disposables should be fine. As an alternative to using nappies and extracting the hydrogel, it is possible to order sodium polyacrylate from Sigma Aldrich.
- Distilled water – if distilled water is not available, tap water can be used but the results are not as spectacular.
- The ice cream tub is for collecting the inside of the nappy and is safer than collecting it over newspaper or similar. If tubs are in short supply, large zip-lock bags can be used. Students put the nappy in the bag, zip it up and manipulate it until all the hydrogel is extracted and then proceed as per the directions.
Procedure
Hair gel
- Put a blob of hair gel onto the petri dish lid. A large teaspoonful is fine.
- Gently sprinkle salt from a spatula over the hair gel.
Disposable nappies
- Cut the middle section out of the nappy – the thicker piece that is designed to absorb the urine. Discard the other piece.
- Make sure the ice cream container is completely dry - wipe it with a paper towel if necessary. Any moisture in the tub stops the experiment from working properly.
- Wear eye protection for the next step. Put the centre piece of the nappy into the ice cream container and gently take it apart. Small white grains should start coming away and this is what you are trying to collect. Keep gently pulling the nappy apart until you have collected as many of the grains as you can. Do not do this roughly or you will lose your product and put a lot of dust and fluff into the air. Avoid breathing in any of the dust.
- Remove and dispose of all the fluff and other parts of the nappy, keeping the grains in the bottom of the tub. They are heavier and fall to the bottom, which makes it easier to separate them out.
- Estimate the volume of the grains.
- Pour them into the large beaker and add about 100 cm3 of distilled water. Stir. Keep adding distilled water until no more can be absorbed and stir between each addition. Estimate the final volume of the hydrogel.
- Add a dessertspoonful of salt and stir.
Teaching notes
This activity can be used to enhance the teaching of ionic and covalent bonding, or hydrogels can be considered as an interesting polymer as well as an example of a smart material. Hydrogels are smart materials because they change shape when there is a change in their environment – in this case it is the change in the concentration of ions.
Students need to have some knowledge and understanding of ionic and covalent bonding, reversible reactions, and acids and bases to understand what is happening.
Hydrogels are polymers that can retain many times their own weight in water. They are often polymers of carboxylic acids that ionise in water, leaving the polymer with several negative charges down its length. This has two effects. First, the negative charges repel each other and the polymer is forced to expand. Secondly, polar water molecules are attracted to the negative charges. This increases the viscosity of the resulting mixture still further as the polymer chain now takes up more space and resists the flow of the solvent molecules around it.
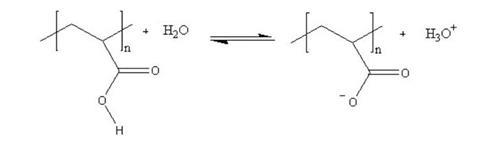
The polymer is in equilibrium with the water around it, but that equilibrium can be disturbed in a number of ways. If the ionic concentration of the solution is increased – eg by adding salt – the positive ions attach themselves to the negative sites on the polymer, effectively neutralising the charges. This causes the polymer to collapse in on itself again. Adding alkali removes the acid ions and moves the equilibrium to the right; adding acid has the opposite effect.
There are a large number of hydrogels and they are sensitive to different pHs, temperatures and ionic concentrations. By using a mix of monomers to create the polymer these characteristics can be fine-tuned.
The hydrogels that are commonly available and are used in this practical activity are sensitive to salt concentration, but do not show much change across the pH range that can be readily investigated in the classroom. However, they do lend themselves very well to a range of investigative practical work. For example, their volume in different amounts of water or in different salt concentrations can be measured. For this type of investigation it is best to use either plant water crystals or to order sodium polyacrylate from Sigma Aldrich – this has a smaller crystal size and gives faster results.
Students should make detailed notes on their experiments, noting changes in volume, colour and any other observations. Some expected observations are given below.
Hair gel
The hair gel shrinks in size very quickly when the salt is added. After a couple of minutes all that is left is some liquid in the petri dish.
Disposable nappies
About 10 cm3 of hydrogel can be extracted from the nappy core. (Exactly how much depends on the make and the size of the nappy.) The hydrogel swells up extremely quickly (much quicker than with plant water storage crystals). It absorbs about 500 cm3 of distilled water giving a very viscous mixture. When salt is added, the viscosity immediately reduces and the mixture is easier to stir. The hydrogel releases the water and settles on the bottom of the beaker.
Additional resources
- The accompanying worksheet – available for download below – explores hydrogels and how they work in more detail.
- There are a variety of resources on water and hydrogels which may be used to run a class investigation based on the (now closed) global experiment on hydrogels, water and wastage.
Downloads
Hydrogels and how they work - worksheet
Editable handout | Word, Size 0.54 mbHydrogels and how they work - worksheet
Handout | PDF, Size 0.61 mb
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet