In this experiment, students make ammonia, investigate its solubility in water and test its alkaline nature
Review some of the key properties of ammonia and give students the opportunity to produce ammonia themselves in this class practical. The experiment provides a useful precursor to the ammonia fountain experiment.
Because ammonia has a strong smell and is poisonous in quantity, pupils should ideally carry out this experiment in fume cupboards. Alternatively, provided that the quantities of reactants are no greater than as stated here, the experiment could be carried out in a well-ventilated laboratory.
Depending on arrangements for setting up and clearing away, the experiments should take around 30 minutes.
Equipment
Apparatus
- Eye protection
- Retort stand, boss and clamp
- Boiling tube with stopper and delivery tube (see diagram below) – this apparatus must be dry
- Boiling tubes, x2 – must be thoroughly dry
- Beaker, 100 cm3
- Beaker, 250 cm3 or larger
- Bunsen burner
- Heat resistant mat
- Spatula
Chemicals
- Ammonium chloride (HARMFUL)
- Calcium hydroxide (IRRITANT)
- Calcium oxide (CORROSIVE) – optional
- Concentrated hydrochloric acid (CORROSIVE)
- Red litmus paper
- Blue litmus paper
- Universal indicator paper
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout. Ammonia gas is TOXIC and DANGEROUS FOR THE ENVIRONMENT and pungent-smelling and must not be inhaled. The experiments must only be carried out in a fume cupboard or in a well-ventilated laboratory.
- Ammonia gas, NH3 (g), (TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC005. Ammonia is a very soluble gas. If the preparation and collection apparatus is not dry, the solubility experiment will not work.
- Ammonium chloride, NH4 Cl(s), (HARMFUL) – see CLEAPSS Hazcard HC009a. A small quantity of ammonium chloride should be provided in a stoppered bottle.
- Calcium hydroxide, Ca(OH)2 (s), (IRRITANT) – see CLEAPSS Hazcard HC018. A small quantity of calcium hydroxide should be provided in a stoppered bottle.
- Calcium oxide, CaO(s), (CORROSIVE) – see CLEAPSS Hazcard HC018. Use lumps or granules of calcium oxide (optional) in a stoppered labelled bottle.
- Concentrated hydrochloric acid, HCl(aq), (CORROSIVE) – see CLEAPSS Hazcard HC047a. Two drops of the acid in the bottom of a stoppered, labelled test tube are sufficient. A small number of prepared tubes could be arranged around the laboratory so that students can access them only under supervision.
Procedure
- In a small beaker, mix 2 spatulas of the ammonium chloride with 2 spatulas of the calcium hydroxide together. The two solids begin to react immediately on mixing.
- Hold a piece of each colour of litmus paper over the mixture and observe the colour change. Test also with a piece of universal indicator paper.
- Transfer the mixture of ammonium chloride and calcium hydroxide into a boiling tube and set up the apparatus as shown in the diagram.
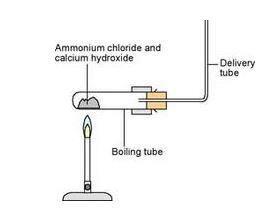
- Optional: put the lump of calcium oxide into the boiling tube containing the ammonium chloride/calcium hydroxide mixture. The calcium oxide will absorb the water produced in the reaction and ensure that the ammonia gas is dry.
- Gently warm the reaction mixture.
- Collect a test tube which contains a few drops of concentrated hydrochloric acid. Remove the stopper from this test tube and hold the open end near the end of the ammonia gas delivery tube. Observe what happens. Replace the stopper on the test tube of hydrochloric acid and return the test tube to its original place.
- Two-thirds fill a large beaker with water. This is needed for step 10.
- Continue to gently warm the reaction mixture. Hold one of the dry boiling tubes in position as shown in the diagram below. Notice that the ammonia is collected with the boiling tube upside down. This is because ammonia is less dense than air.
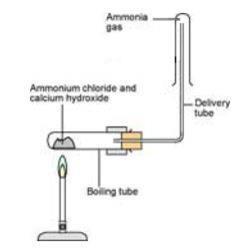
- Test around the open end of the collecting boiling tube with universal Indicator paper to check that the collecting tube is full of ammonia.
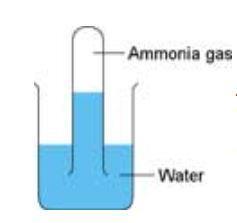
- Hold the tube of ammonia upside down then quickly put it, mouth still downwards, into water in a beaker. The ammonia dissolves in the water and the level of the water should rise up inside the test tube. If you want to try this a second time, use a fresh dry boiling tube.
Teaching notes
Ammonia is a very soluble gas. 1 cm3 of water dissolves about 800 cm3 of ammonia at room temperature. A few drops of water will easily dissolve a test tube of ammonia. This is why it is essential that the apparatus is dry.
The reaction to produce ammonia also produces water. The purpose of the calcium oxide is to help to prevent this water from coming out of the delivery tube as water vapour.
The equation for the generation of ammonia is:
2NH4Cl(s) + Ca(OH)2(s) → CaCl2(s) + 2NH3(g) + 2H2O(g)
Calcium oxide reacts with water to produce calcium hydroxide:
CaO(s) + H2O(l) → Ca(OH)2(s)
Ammonia is one of the very few common alkaline gases. When it dissolves in water it reacts reversibly according to the equation:
NH3(g) + H2O(l) → NH4+(aq) + OH–(aq)
There is generally enough water on the surface of indicator papers to dissolve ammonia without having to moisten the paper.
The white fumes (‘smoke’) produced with the hydrogen chloride given off by the concentrated hydrochloric acid consist of fine particles of solid ammonium chloride.
The equation is:
NH3(g) + HCl(g) → NH4Cl(s)
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet