NMR is particularly useful in the identification of the positions of hydrogen atoms (1H) in molecules.
The NMR spectrum of ethyl benzene, C6 H5 CH2 CH3, is shown below.The frequencies correspond to the absorption of energy by 1H nuclei, which are protons. Notice that there are three major peaks of differing heights.
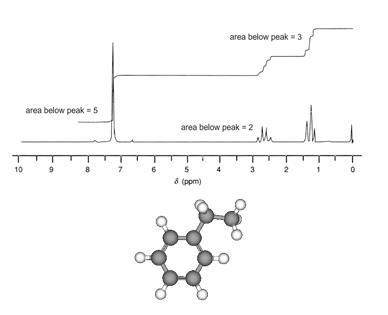
Each peak corresponds to a hydrogen atom in a different molecular environment. The area under each peak is proportional to the number of that type of hydrogen atom in the molecule.
The largest peak
Corresponds to the five atoms in the benzene ring [C6 H5].
The second largest
Corresponds to the three hydrogen atoms in the methyl group [CH3].
The third peak
Corresponds to the two hydrogen atoms in the methylene group [CH2].
The hydrogen atoms in a particular type of environment have similar positions in an NMR spectrum. Normally, this position is measured as a chemical shift from a fixed reference point. The reference point normally used is the absorption of a substance called tetramethylsilane (TMS), which has the formula (CH3)4 Si.
Advanced chemistry – the NMR spectrum of ethanol (low resolution)
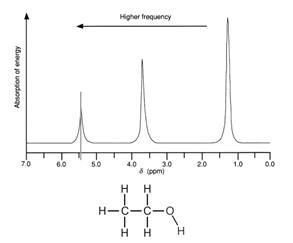
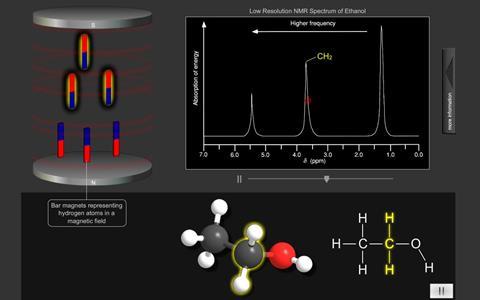
The simplified proton NMR spectrum of ethanol enables the hydrogen atoms to be easily identified. Notice also that spectra also show the integration of the peaks (the area under each peak).
Thus in the spectrum opposite, the smallest peak represents the single H in the OH group (integration of 1)
the middle peak represents the H in the CH2 group (integration of 2)
the largest peak represents the H in the CH3 group (integration of 3)
If the spectrum of ethanol is recorded as a high-resolution spectrum, more detail is apparent and the peaks appear as singlets, doublets, triplets, quartets etc.
Advanced chemistry – the NMR spectrum of ethanol (high resolution)
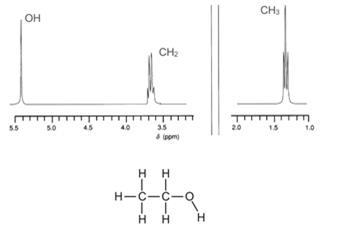
If the spectrum of ethanol is recorded as a high-resolution spectrum, more detail is apparent and the peaks appear as singlets, doublets, triplets, quartets etc.
The sets of peaks are due to interaction of protons from neighbouring groups. Thus, in the spectrum of ethanol, the CH3 group affects the CH2 group and vice versa. The phenomenon is known as spin-spin coupling and provides essential information for a skilled NMR technician to interpret a spectrum.
Advanced chemistry – spin-spin coupling
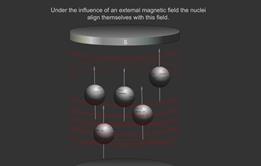
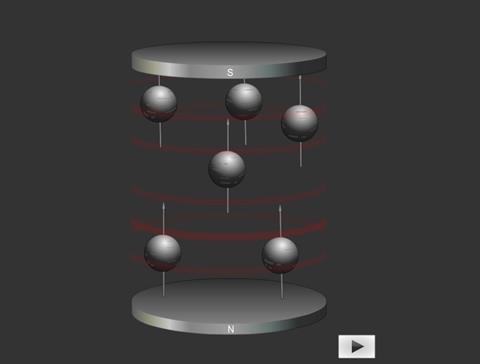
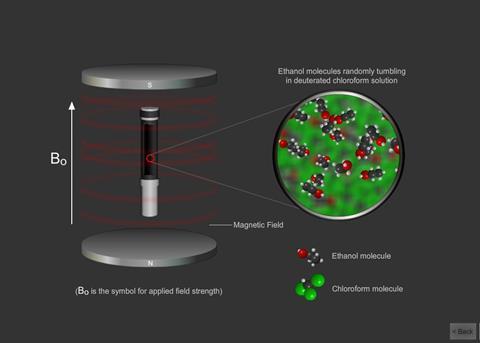
In a molecule the nucleus of an atom can induce in the electrons of the chemical bonds attached to it a very weak magnetic moment. This affects the magnetic field at a neighbouring atom’s nucleus.
The interaction is known as coupling and this causes the peaks to be split into a number of smaller ones. Protons can usually interact with other protons that are up to three bonds away.
Protons in the same chemical environment do not show coupling with each other.
View the following animations for a more detailed explanation of spin-spin coupling:
Spin-spin coupling 1: Spinning charges can be regarded as minute (atomic) bar magnets
Spin-spin coupling 2: Atoms giving rise to chemical shifts in the low resolution NMR spectrum of ethanol
Spin-spin coupling 3: High resolution NMR spectrum and an explanation of spin-spin coupling.
Advanced chemistry – the NMR spectrum of iodoethane CH3 CH2 I
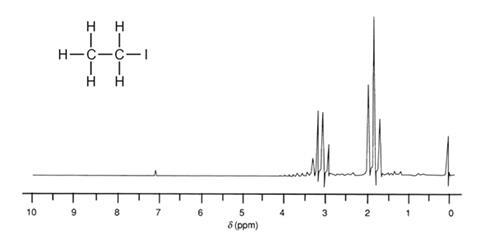
The CH3 protons produce a peak at δ 1.8 but, instead of a single peak, a triplet is produced. This is because the CH3 protons couple with the adjacent two CH2 protons.
The CH2 protons produce a peak at δ 3.2 but, instead of a single peak, a quartet is produced. This is because the CH2 protons couple with the adjacent three CH3 protons.
In general, if there are ‘n’ protons three bonds away from the resonating group, the absorption will be split into a multiplet of n+1 lines.
Downloads
Spin-spin coupling 1
Simulation | Flash, Size 18.59 kbSpin-spin coupling 2
Simulation | Flash, Size 0.14 mbSpin-spin coupling 3
Simulation | Flash, Size 0.13 mb
Nuclear magnetic resonance (NMR) spectroscopy
Discover how nuclear magnetic resonance (NMR) spectroscopy works, with this series of topics breaking down the fundamental theory. Covering the electronic environment of atoms right up to demonstrating the practical identification of molecules. Includes examples and interactive simulations to aid understanding.
- 1
 Currently
reading
Currently
reading
Hydrogen NMR





















No comments yet