The first of three steps into the preparation of paracetamol
Paracetamol can be made in three steps from phenol
The nitration of phenol
Health, safety and technical notes
- Wear eye protection.
- Protective gloves should be worn when handling phenol.
- Read our standard health and safety guidance
- Sodium nitrate(V) - oxidising agent, keep away from flammable material. See CLEAPSS Hazcard HC093
- Concentrated sulfuric acid - very corrosive dense liquid, dehydrating agent, add very slowly with constant stirring to water whilst cooling. See CLEAPSS Hazcard HC098a
- Phenol - toxic by ingestion and skin absorption. It can cause severe burns. Take care when removing phenol from the bottle because the solid crystals can be hard to break up.See CLEAPSS Hazcard HC070a
- Wear protective gloves and work in a fume cupboard.
- 2- and 4-nitrophenols - harmful and irritant, will stain skin, wear protective gloves when handling. See CLEAPSS Hazcard HC070a
Chemicals (per group)
- Concentrated sulfuric acid
- Sodium nitrate(V)
- Solid phenol
- Hydrochloric acid (0.5 mol dm-3)
Apparatus and equipment (per group)
- 3-necked round bottom flask
- Magnetic stirrer
- Ice bath
- Thermometer
- Quickfit@ distillation apparatus (still head, condenser, stoppers)
- Dropping funnel
- Spatulas
- Access to a balance
- Access to a fume cupboard
Method
- Place a 3-necked round bottom flask in an ice water bath and place a thermometer into one of the necks. Place 15g of sodium nitrate(V) into the flask, add 40 cm of water and stir.
- Cautiously add concentrated sulfuric acid (13.6 cm3; 25 g) to the stirred solution.
- Slowly add solid phenol (9.4g, 0.1 mol) at such a rate that the temperature of the solution does not rise above 20◦C (about a half a spatula at a time over about 20 minutes) and then stir, preferably with a magnetic stirrer, for 2 hours.
- Remove the thermometer. Decant off the supernatant liquid and add water (25-30 cm3) to the residue. Put a dropping funnel into one of the necks and a still head and condenser into another. Insert a stopper in the third.
- Heat the mixture, and distil off one of the components with the steam. At the same time add water to the mixture through the dropping funnel at a similar rate.
- Stop the distillation once the product has stopped coming over, then filter the distillate to give crystals of the 2-nitrophenol isomer.
- Cool the residual solution in the distillation flask, then filter off the other solid isomer (4-nitrophenol) and recrystallise it from 0.5 mol dmp3 hydrochloric acid.
Questions
- Why does the reaction give a mixture of isomers? What other product might you get?
- Why does the nitration need only mild conditions, whilst benzene requires concentrated H2 S04/HNO3?
- What happens when you add concentrated sulfuric acid to sodium nitrate(\/)?
- The products can be separated by a simple steam distillation procedure. The 2-nitrophenol product is volatile in steam but the 4-nitrophenol is not. Why?
- The two products can be separated by chromatography. The 2-nitrophenol has less affinity for silica (ie runs faster) than the 4-nitrophenol. Why?
- What are the melting points of the pure products? Why is one lower than the other?
- Dissolve each product in water - which solution is more acidic and why?
- Look up the melting points of the following pairs of compounds (be careful how you interpret the names).
- 3-methyl-2-nitrophenol
- 3-methyl-4-nitrophenol
- 5-methyl-2-nitrophenol
- 3-methyl-+nitrophenol
- 5-fluoro-2-nitrophenol
- 3-f1 uoro-4-nitrophenol
What do you notice about the melting points?
Answers
- It is possible to get 2- and 4- substitution of the ring due to effective charge stabilisation at both these sites. The -OH group activates the ring preferentially at the 2- and 4- positions and therefore directs substituents to these positions (rather than to the 3- and 5- positions which are not activated). Another product could be the di-substituted form:
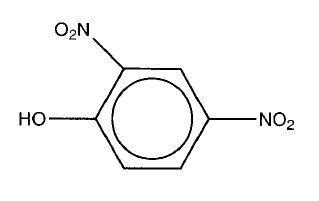
- Phenol activates the ring to electrophilic attack because the lone pair on the oxygen pushes electron density onto the ring. This means nitration is easier and does not require such harsh conditions.
- H2SO4 + 2NaNO3 -+ Na2SO4 + 2HNO4. Then in excess sulfuric acid: HNO3 + H2SO4 -+ NO2+ + HSO4- + H2O. This is sometimes seen as: HNO3 + 2H2SO4 + NO2+ + 2HSO4- +H3+O
- 4-Nitrophenol is better at hydrogen bonding with other molecules of 4-nitrophenol and/or water so it is less volatile. 2-nitrophenol has intramolecular hydrogen bonding and consequently, fewer intermolecular hydrogen bonds with other molecules, so is more volatile.
- 2-Nitrophenol is less polar due to intramolecular hydrogen bonding.
- Melting point of pure 4-nitrophenol = 113.8 ◦C. Melting point of pure 2-nitrophenol = 45.1 ◦C. 4-Nitrophenol has a higher melting point due to intermolecular hydrogen bonding, holding the molecules together in the crystal.
- 4-Nitrophenol is more acidic. The hydrogen on the OH of the 2-nitrophenol is stabilised by intramolecular hydrogen bonding and so it is not released as readily
- This question gives three examples of pairs of nitro phenols where the 2-isomer has a lower melting point than the 4-isomer. The melting points can be found in a general chemical supplies catalogue such as Aldrich - Handbook of Fine Chemicals and Laboratory Equipment
- 3-methyl-2-nitrophenol m.p. 35-39 ◦C
- 3-methyl-4-nitrophenol m.p. 127-129 ◦C
- 5-methyl-2-nitrophenol m.p. 53-56 ◦C
- 3-methyl-4-nitrophenol m.p. 127-129 ◦C
- 5-fluoro-2-nitrophenol m.p. 35-37 ◦C
- 3-f I uoro-4-nitrophenol m .p. 93-95 ◦C
It is an interesting exercise in seeing how names and locants change in that 3- and 5-substituents are in fact the same position with respect to the phenol -OH.
Downloads
The preparation of paracetamol - editable
Word, Size 0.19 mbThe preparation of paracetamol
PDF, Size 0.34 mb
Paracetamol book
- 1
- 2
- 3
- 4
- 5
 Currently reading
Currently readingThe preparation of paracetamol
- 6
- 7
- 8
- 9
























1 Reader's comment