How can we form aminde’s?
Use this class practical to explore the formation of an amide
Health, safety and technical notes
- Read our standard health and safety guidance
- Wear eye protection.
- 4-aminophenol - irritant. See CLEAPSS Hazcard HC070b
- Ethanoic anhydride - corrosive, causes burns, flammable, the vapour will irritate the eyes and the respiratory system. Use in a fume cupboard. See CLEAPSS Hazcard HC039
- Paracetamol - do not ingest.
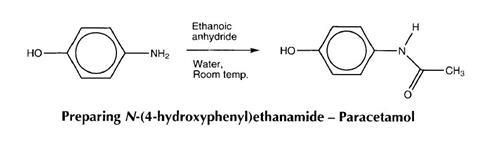
Preparing N-(4-hydroxyphenyl)ethanamide - Paracetamol
Chemicals (per group)
- 4-Aminophenol
- Ethanoic anhydride
Apparatus and equipment (per group)
- Two 50 cm3 conical flasks
- One 100 cm3 beaker
- Tripod and gauze
- Bunsen Burner
- Thermometer (-10◦C to 110◦C)
- Buchner flask, funnel and filter papers
- Melting point apparatus
- Glass rod or magnetic stirrer
- One 10 cm3 and one 50 cm3 measuring cylinder
- Clamp and stand
- Access to oven and fume cupboard
- Ice and water supply
- Water pump
- Eye protection
Method
- Place 1.0 g of 4-aminophenol and 9 cm3 of distilled water in a 50 cm3 conical flask and stir briskly at room temperature, in order to suspend the solid in the water.
- In a fume cupboard, add 1.1 cm3 (1.17 g) of ethanoic anhydride to the stirred suspension and gently shake to mix. The solid will dissolve after about 30 seconds. Continue shaking and a precipitate will form after 2 minutes.
- After 10 minutes the solid should be filtered off under suction, washed with a little cold water and dried (0.83g; 60%).
- The product may be purified by crystallisation from distilled water. Dissolve the crude product in the minimum of distilled water at about 80◦C (you will probably require about 15 cm3).
- Allow the clear solution to cool slowly to room temperature and collect the recrystallised product by suction filtration, washing with 5 cm3 of ice-cold distilled water.
- Dry the recrystallised product either between filter papers or by gently warming in an oven, and determine the yield.
- Determine the melting point of the dry, recrystallised product
The melting point should be 169-171◦C.
Questions
- Why is the product insoluble in aqueous ethanoic acid, but the starting material is soluble
- If the reaction is done in dilute hydrochloric acid rather than water, the product is
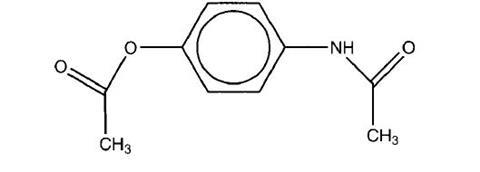
Why is this?
Answers
1. Amides are not basic because the lone pair on nitrogen is delocalised over the carbonyl group. They are therefore not soluble in acid. Amines are basic and do dissolve in acid.
2. At low pH the NH, is protonated and allows the OH to react with ethanoic anhydride. The ester that is produced is still soluble in the acid due to -NH, protonation, but due to partial dissociation, this group is eventually ethanoylated and thrown out of the solution. At higher pH, the NH, is not protonated and rapidly gets ethanoylated and thrown out of solution before the -OH has a chance to react.
Paracetamol book
- 1
- 2
- 3
- 4
- 5
- 6
 Currently reading
Currently readingThe formation of an amide
- 7
- 8
- 9

























No comments yet