Chromatography techniques are used a great deal in industry, the chemical sciences included
You have probably used a simple chromatography experiment as part of your earlier studies to separate the dyes in a coloured ink. The same technique can be used to separate substances which are not dyes but in such experiments the chromatogram must be developed to show up the various different substances that have been separated.
A spot of the compound being investigated is placed on a chromatography plate, and a spot of a pure manufactured sample of the same substance is placed next to it. The plate is then allowed to stand in a suitable solvent, which travels up the plate. If the compound to be identified leaves exactly the same pattern on the chromatography plate as the known pure compound it is reasonable to conclude that they are the same. However, if extra spots are observed as well as the characteristic pattern of the known compound, then impurities are likely to be present in the sample.
In the experiment both crude samples of 2-nitrophenol and 4-nitrophenol are compared with known samples.
Health, safety and technical notes
- Wear eye protection.
- Read our standard health and safety guidance https://rsc.li/4bCW4OT
- Ethyl ethanoate - volatile, highly flammable, keep away from flames, irritant, do not inhale vapour.
- Cyclohexane - volatile, highly flammable, keep away from flames, harmful, do not inhale vapour.
- Iodine - harmful by inhalation or skin contact.
- Short wave UV - may cause skin cancer and eye damage. Do not observe directly. The viewer should be screened from direct radiation.
Chemicals (per group)
- 2-Nitrophenol
- 4-Nitrophenol
- Ethyl ethanoate
- Cyclohexane
- Iodine
- Solid sodium hydrogencarbonate
Apparatus and equipment (per group)
- Four test-tubes
- Marker pen and pencil
- Access to fume cupboard
- Capillary melting point tubes
- TIC plate (or chromatography no. 1 paper)
- Chromatography tank (or 1 dm3 beaker)
- Measuring cylinders
- Spatula
- Access to UV lamp screened from direct view
- Eye protection
Method
- Make sure that you do not touch the surface of the tlc plate with your fingers during this activity. Handle the plate only by the edges and use tweezers if possible.
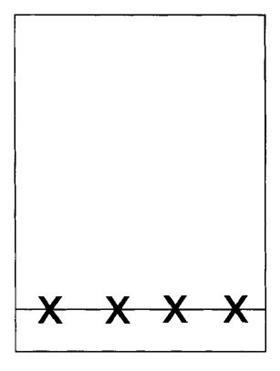
- Take a tlc plate and using a pencil (not a ball-point or felt tip pen) lightly draw a line across the plate about 1 cm from the bottom. Mark four equally spaced points on this line.
- Place small amounts (about 1/3 of a spatula measure) of your crude 2-nitropheno1, your crude 4-nitrophenol and the commercial samples of these in four separate test-tubes. Label the test-tubes so that you know which is which.
- Add 1 cm3 of ethyl ethanoate to the test-tubes to dissolve the samples. If possible do this in a fume cupboard.
- Use clean capillary tubes to spot each of your four samples onto the tlc plate. Allow the spots to dry and then repeat. The spots should be about 1-2 mm in diameter.
- After all the spots are dry, place the tlc plate in the chromatography tank making sure that the original pencil line is above the level of the developing solvent - ethyl ethanoate:cyclohexane 1 :4. Put a lid on the tank and allow to stand in a fume cupboard until the solvent front has risen to within a few millimetres of the top of the plate.
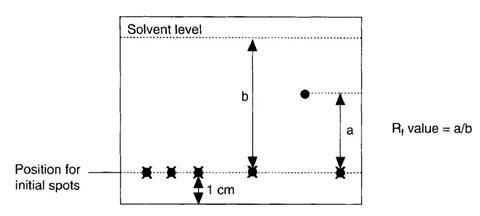
- Remove the plate from the tank and quickly mark the position of the solvent front. Allow the plate to dry.
- Observe the plate under a short wavelength UV lamp and lightly mark with a pencil any spots observed.
- Carefully place the plate in a jar or beaker containing a few iodine crystals. Put a cover on the jar and wait for the spots to appear. Do this in a fume cupboard if possible.
Results
Draw a diagram to show which spots appeared under UV light and which appear with iodine.
Determine the R, value of the samples using the expression
R, = distance moved by sample / distance moved by solvent
Questions
- Write a short paragraph explaining why some substances move further up the tlc plate than others and how the results are made visible.
- What conclusions can you draw about the nature of the four samples tested?
Answers
- Less polar substances move further up the plate because they have less affinity for polar silica - results made visible using ultraviolet light, iodine staining or potassium permanganate staining.
- Dependent on results.
Downloads
Using thin-layer chromatography to investigate the reactions - editable
Word, Size 85.04 kbUsing thin-layer chromatography to investigate the reactions
PDF, Size 0.17 mb
Paracetamol book
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
 Currently reading
Currently readingUsing thin-layer chromatography to investigate the reactions

























No comments yet