What to know before you explore paracetamol
Paracetamol is a common compound
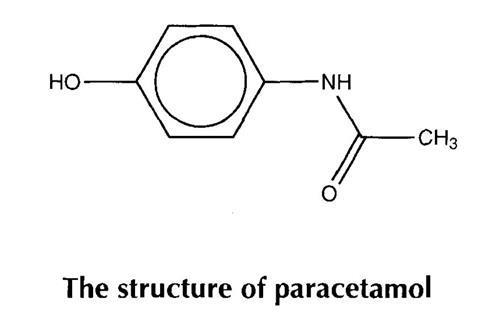
Paracetamol is a very widely used medicine. It is a mild painkiller and reduces the temperature of patients with fever. These actions are known respectively as analgesic and antipyretic.
There are currently more than 90 common products containing paracetamol which are available over the counter from British pharmacies. Many of them are sold as treatments for the relief of cold and influenza and they can be bought in a number of different formulations. Paracetamol is a relatively safe drug but toxic side effects have been observed with high doses greater than 10-15 g.
This toxicity is due to the chemical structure of the compound and the way our bodies break it down. It is metabolised to a reactive intermediate at high doses. Pure paracetamol is a white crystalline solid which melts at 169-171 “C. Its solubility in cold water is 1.43 dl00 cm3 but it is much more soluble in hot water (5 d100 cm3) and in ethanol (14 d100 cm3).
The history of paracetamol
At the University of Strassburg in the 1880s Professor Kussmaul, of the Department of Internal Medicine, asked two assistants to give naphthalene as a treatment for intestinal worms.
The medicine had little effect on worms, but one patient had a great reduction in fever temperature. It was found that this patient had, in fact, been given acetanilide instead of naphthalene due to a mistake at the pharmacy!
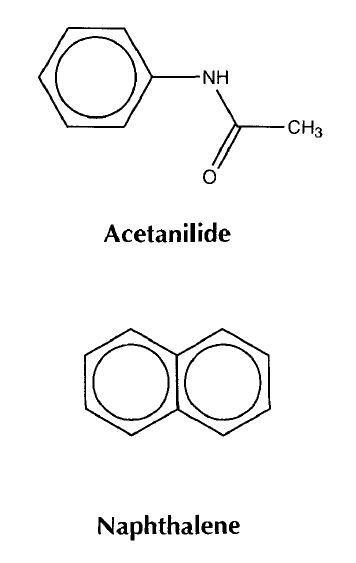
The young assistants quickly published the discovery of this new antipyretic (fever reducing drug). It was soon in production and remained in use for several years because it was so cheap to produce. However, it had a serious side effect involving the deactivation of some of the haemoglobin in red blood cells.
The publication of news about acetanilide immediately spurred a chemist at Bayer’s dyeworks to make some derivatives:
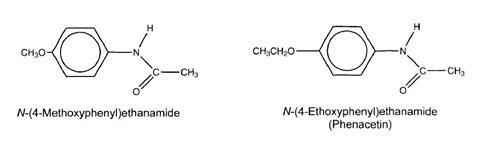
These were both found to be antipyretic and N-(4-ethoxyphenyl)ethanamide was less toxic than acetanilide itself. It was promptly marketed as ’Phenacetin’ and has remained in use ever since. However, restrictions have been placed on its use due to kidney damage in long-term users.
Many medicines were synthesised to try to improve on phenacetin and as early as 1893 Joseph von Mering made paracetamol.
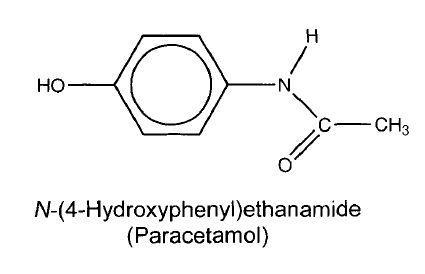
He found it to be an effective antipyretic and analgesic, but wrongly thought that it caused the same haemoglobin problem as acetanilide.
It was not until the 1940s that paracetamol was reinvestigated after it was found present in patients dosed with phenacetin. In 1953 paracetamol was marketed by Sterling-Winthrop Co., and promoted as preferable to aspirin since it was safe to take for children and people with ulcers. However, it causes liver damage from chronic use.
Paracetamol is rapidly formed in the guts of people who take phenacetin. It is the major metabolite (decomposition product) and it is likely that the antipyretic and analgesic effects of phenacetin were in fact due to paracetamol.
There are some suggestions that the toxic effects of phenacetin were due to a minor metabolite - the N-oxide.
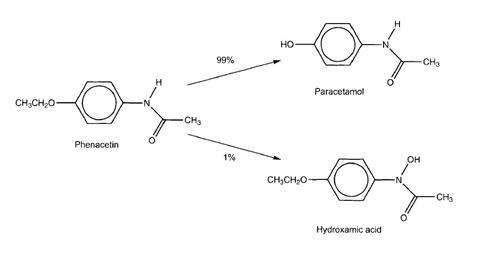
Phenacetin is metabolised to two compounds. One involves the removal of the ethyl (CH,CH,-) substituent from oxygen. The second involves the replacement of the hydrogen atom on nitrogen by a hydroxyl (-OH) group. This type of compound is called a hydroxamic acid. Hydroxamic acids bind strongly to metal ions. This action may contribute to the toxicity.
Methods of establishing the safety and efficacy of medicines
At the turn of the 20th century the discovery and testing of medicines was a largely haphazard process with the pioneers of high quality science such as Pasteur and Erhlich being exceptions. As in the history of paracetamol, new compounds were given to patients almost immediately after synthesis or discovery.
In contrast, the development of modern medicines is a long and expensive process typically taking 10-12 years. Chemistry is used at all stages to develop the synthesis and determine purity. Potential patients need to know at least four things about a new medicine before they take it. Does it work? Is it safe? How much do I take? How often do I take it?
The answer to the first question is initially investigated in isolated enzyme or cellular systems before being trialed in animal models and ultimately in volunteer patients.
Clinical trials are used in the later stages to see if a new medicine works in one set of patients compared to the effects of a placebo on another group.
The only way to determine safety and dosage regimes are to use animal models before human volunteers take the medicine.
Task
Consider the ethical considerations of testing new medicines on animals.
Put this in context
Find out about the role of a director of medicinal chemistry who is at the forefront of medicinal research.
Downloads
The quantitative analysis of various formulations of paracetamol
PDF, Size 0.21 mbThe quantitative analysis of various formulations of paracetamol - editable
Word, Size 0.16 mb
Paracetamol book
This book consists of seven freestanding activities that can be used in a wide range of teaching and learning situations in both academic and vocational courses.
- 1
 Currently
reading
Currently
reading
Background information
- 3
- 4
- 5
- 6
- 7
- 8
- 9


































No comments yet