Use this practical to demonstrate the chemistry behind rechargeable batteries, using a lead–acid accumulator cell
Some electrochemical cells are rechargeable – the electrode reactions are reversible and the process can be repeated many times. Such cells can be used to store electricity. The most common type of heavy duty rechargeable cell is the familiar lead-acid accumulator (‘car battery’) found in most combustion-engined vehicles.
This experiment can be used as a class practical or demonstration. Students learn how to construct a simple lead–acid cell consisting of strips of lead and an electrolyte of dilute sulfuric acid. The cell should then be charged for different lengths of time, before being discharged through a light bulb. Students measure the time the bulb remains lit, plotting a graph of this time against the charging time to show the relationship between the electrical energy put into the cell and the energy released.
Without going into the detail of the electrode reactions, this experiment can be used as a demonstration or class exercise to investigate a reversible electrochemical cell in the context of alternative energy sources for vehicles, or energy storage. To date the lead-acid accumulator has proved to be the only widely used source of energy for electrically powered vehicles. Other types of electrochemical cell, especially fuel cells, are now being developed and tested on the road. Some of the criteria for a commercially viable cell can be discussed.
At advanced level the electrode processes could be outlined in more detail as examples of redox reactions that can be reversed many times in an electrochemical cell. Although car battery testing using the density of the electrolyte has become less common, its relationship to the overall cell reactions, on charging and discharging the lead accumulator, could also be pointed out.
Time required should be 20–30 minutes, depending on how many readings are taken.
Equipment
Apparatus
- Eye protection
- Beakers, 100 or 150 cm3, x2
- Low voltage DC supply, 2–4 V, or suitable battery
- Torch bulb, 1.25 V, in holder
- Crocodile clips, x2–4, as needed
- Connecting leads, x2
- Stopclock or watch
Chemicals
- Dilute sulfuric acid, 0.5 M (IRRITANT), about 100 cm3
- Lead foil electrodes (TOXIC, DANGEROUS FOR THE ENVIRONMENT) (about 2 cm x 8 cm), x2 (see note 6 below)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Lead foil, Pb(s), (TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC056.
- Dilute sulfuric acid, H2 SO4 (aq), (IRRITANT) – see CLEAPSS Hazcard HC098a and CLEAPSS Recipe Book RB098.
- Lead(IV) oxide, PbO2 (s), (TOXIC, DANGEROUS FOR THE ENVIRONMENT) is produced as a product on the (–) electrode – see CLEAPSS Hazcard HC056.
- The lead electrodes should be cut to size so that they can be folded over the rim of the beaker and the crocodile clips attached, so as to grip the beaker rim and the lead foil together. Care must be taken not to allow the electrodes to touch once the cell is assembled, or for the electrolyte level to bring it into contact with the crocodile clips.
Procedure
- Assemble the cell as shown in the diagram and connect it to the DC source. Note which electrode is (+) and which is (–).
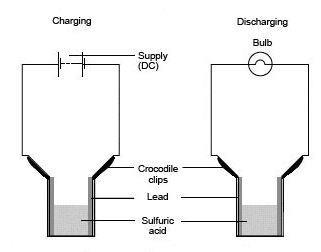
- Pour sufficient dilute sulfuric acid electrolyte into the cell to fill it to within 1 cm of the crocodile clips.
- Switch on the DC source and, if possible, adjust the voltage to 3–4 V. Allow the current to pass for three minutes.
- Disconnect the power supply from the cell. At this point the lead electrodes may be removed for examination (demonstration mode only). One should be bright, the other covered with a dark brown deposit of lead(IV) oxide (TOXIC, DANGEROUS FOR THE ENVIRONMENT). Replace the electrodes in the electrolyte.
- Connect the electrodes to the light bulb and start the stop clock. The bulb will light up then gradually fade. Note the time it takes to go out.
- Replace the light bulb with the DC power source, making sure that the electrodes are connected to the same DC terminals as at the start. Pass the current for four minutes this time. Disconnect the power supply and time how long it takes to discharge the cell using the light bulb.
- Repeat steps 3–6 a few more times, each time increasing the charging time by a minute and recording the time it takes for the cell to discharge.
- Plot a graph of discharge time (y-axis) vs charging time (x-axis).
Teaching notes
Students should be able to identify which way electrons are flowing in the cell when it is charging and discharging from the electrode polarities. At advanced level this could be linked to the electrode reactions below, which assume an initial layer of insoluble lead(II) sulfate on the electrodes after immersing the lead in the acid.
During charging (electrode signs as in charging circuit):
(+) electrode: PbSO4(s) + 2H2O(l) → PbO2(s) + 4H+(aq) + SO42–(aq) + 2e–
(–) electrode: PbSO4 (s) + 2e– → Pb(s) + SO42–(aq)
Discharging (electrode signs as for cell):
(+) electrode: PbO2(s) + 4H+(aq) + SO42–(aq) + 2e– → PbSO4(s) + 2H2O(l)
(–) electrode: Pb(s) + SO42–(aq) → PbSO4(s) + 2e–
The overall, reversible cell reaction is therefore:
PbO2 (s) + 4H+ (aq) + 2SO42- (aq) + Pb(s) ⇌ 2PbSO4 (s) + 2H2 O(l)
Thus during charging the sulfuric acid concentration rises, and during discharge it falls. A side reaction which may result from over-charging is the liberation of hydrogen gas at the (–) electrode, resulting from the reduction of H+(aq) ions. This has caused explosions in the past when the electrolyte level in batteries has been investigated with the aid of a lighted match!
The advantages of this cell reaction for use in a commercial battery could be discussed, eg the formation of insoluble lead or lead compounds on the electrodes during charge and discharge, the only changes in the electrolyte being a change in concentration. Commercial cells need to be robust, cheap to construct and, for certain applications, able to sustain large currents. The lead-acid accumulator fulfils all these criteria, but has the disadvantage of being very heavy.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















1 Reader's comment