A question and answer sheet that tests learner’s knowledge of redox equilibria
The topics covered in this Starter for ten activity are: redox reactions, standard electrode potentials, calculations involving electrochemical cells, using EƟ values to predict reactions, appplications of electrochemical cells.
Example questions
Faisal has written the following notes on redox reactions in preparation for his AS exams. However there are a few mistakes, many of which are commonly seen in exam answers. Help Faisal learn from his mistakes by correcting the errors so that he has an accurate set of notes to revise from.

We can measure how readily something gives away electrons by measuring its standard electrode potential, E⦵.
1. Standard electrode potentials are measured by connecting a half cell containing the equilibrium, the potential of which is to be measured to a standard hydrogen electrode at 298 K.
(a) Label the diagram below showing the standard hydrogen electrode.
(b) Complete the diagram to show the complete cell you would use if you wished to measure E⦵ for a zinc electrode.
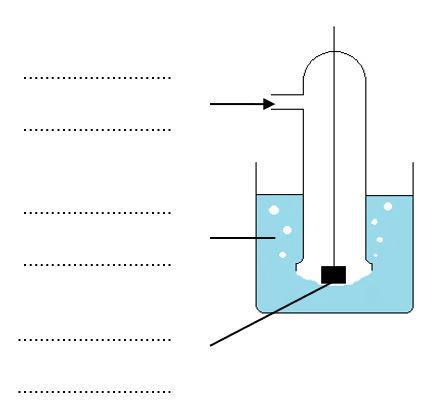
2. Cells can be represented in shorthand form using a series of standard conventions.
(a) Match up the symbol to its meaning when used to represent an electrochemical cell;
| Shows a salt bridge
|| Indicates a phase boundary
(b) For each half cell, the species in the highest oxidation state in the redox equilibrium is written next to the salt bridge.
Use this convention to complete the shorthand representation of the cells produced when half cells containing each of the equilibria below are connected to a standard hydrogen electrode.
(i) Fe2+(aq) + 2 e– ⇌ Fe(s); Pt | H2(g) | H+(aq) ||
(ii) MnO4-(aq) + 1 e– ⇌ MnO42-(aq); Pt | H2(g) | H+(aq) ||
Notes
A full version of this question and answer sheet is available from the ‘downloads’ section below. An editable version is also available.
Downloads
Redox equilibria - editable
Word, Size 0.5 mbRedox equilibria
PDF, Size 0.55 mb
Starters for 10: Advanced level 2 (16–18)

This chapter in our Starters for ten series covers: kinetics, equilibria, acids and bases, carbonyl chemistry, aromatic chemistry, compounds with amine groups, polymers, structure determination, organic synthesis, thermodynamics, periodicity, redox equilibria, transition metal chemistry, and inorganics in aqueous solution.
















































No comments yet