Try this practical to reduce copper(II) oxide to copper using hydrogen, revealing their positions in the reactivity series
In this experiment, students heat copper(II) oxide in the presence of hydrogen gas to extract copper. The copper(II) oxide reacts with hydrogen and is reduced, producing copper and water. This can be used to show the relative positions of copper and hydrogen in the reactivity series, and can also be used for percentage yield-type calculations.
This practical involves some specialist microscale equipment such as a Comboplate® with lids and a microburner. If this equipment is available in your institution this is a good practical to do. It is probably not worth buying the equipment in specially. If students are familiar with the equipment then this takes only a few minutes to set up and run. For students who are not it could take a lot longer.
Equipment
Apparatus
- Eye protection
- Comboplate®, 1 (see note 8 below)
- Syringe, 2 cm3
- Lid number 1
- Lid number 2
- Short lengths silicone tubing, x2
- Piece of glass tubing, 10 cm, of a width to match the silicone tubing
- Matches
- Access to a balance which reads to two decimal places (if students are calculating percentage yield)
Chemicals
- Copper(II) oxide (HARMFUL, DANGEROUS FOR THE ENVIRONMENT), ~ 0.5 g
- Magnesium ribbon, 3 cm
- Dilute hydrochloric acid, 2 M (IRRITANT), about 2 cm3
- Microburner filled with ethanol (HIGHLY FLAMMABLE), or industrial denatured alcohol, IDA (HIGHLY FLAMMABLE, HARMFUL) (see note 6 below)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Copper(II) oxide, CuO(s), (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC026.
- Dilute hydrochloric acid 2 M, HCl(aq) – see CLEAPSS Hazcard HC047a.
- Magnesium ribbon, 3 cm – see CLEAPSS Hazcard HC059A.
- Ethanol or IDA is highly flammable – see CLEAPSS Hazcard HC040A. Keep the microburner upright and blow it out when you have finished. Students should be warned about the dangers of the ethanol in the microburners. They should keep the burner upright and should not take off the lid. Ethanol can catch fire very easily and produces highly flammable vapour. To minimise any risk, ensure that the burners are filled prior to the lesson. Students need to light their burners and the easiest way to do this is with matches. It is probably advisable to have a box of matches per bench, to know how many you have and to count them in and out at the start and end of the session. Having a limited number of matches in each box reduces the potential for mischief. As an alternative, have a lit Bunsen burner or two in the laboratory and have students use splints to light their burners. However, they can be a little tricky to light this way.
- There is a danger of the glass breaking when inserting the glass tube into the silicone tubing. Warn students of this and show them how to hold the glass tube near the end that they are attaching to the silicone tube so as to minimise the risk. Technicians can do this.
- Microscale equipment can be sourced on the internet. Philip Harris has a range of suitable equipment.
Procedure
- Attach a piece of silicone tubing to both lids.
- Half-fill well F1 with tap water and attach lid number 2.
- Gently coil the magnesium ribbon to fit into well F6 and attach lid number 1.
- Fill well E6 with hydrochloric acid.
- Dip the end of the glass tubing into the pot of copper oxide and collect some in the end of the tube. Gently tap the tube to get the copper oxide more or less into the middle.
- Holding the glass tube near the end you are attaching, fix each end to the silicone tubing attached to the lids (see diagram below).
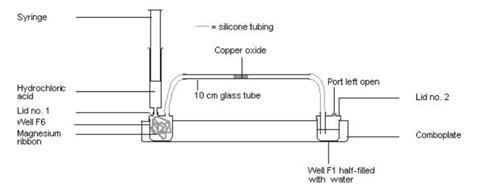
- Fill the syringe carefully with acid from well E6, attach the syringe to the lid on well F6, and add a drop or two to the magnesium to get it fizzing. You will see if the hydrogen is flowing through the system as it produce bubbles in the water in well F1. You need to add enough acid throughout the experiment to keep a slow but steady stream of bubbles.
- Light the microburner and begin to heat the copper oxide in the tube. Keep heating for a minute or two and watch for changes occurring in the tube. When there are no more changes, stop heating, wait for a moment, and then try to light the gas coming out of well F1. Blow out the microburner and detach the lids from the Comboplate®.
- When the tube has cooled, tap the end gently on a piece of rough paper to extract the product.
Teaching notes
The magnesium reacts with the acid to produce hydrogen. As the copper oxide is heated it reacts with the hydrogen and is reduced, producing copper and water. The black copper oxide at the start turns to orange copper (this could be tested with a circuit tester once it has been removed from the tube) and a liquid which is water can be seen at the end of the tube. Lighting the gas coming out of the lid should convince students that it is hydrogen as it produces the expected ‘squeaky pop’.
Copper oxide + hydrogen → copper + water
CuO + H2→ Cu + H2 O
If you want students to calculate the percentage yield, they need to weigh the glass tube at the start, with the copper oxide in it, and at the end with the copper. If there appears to be a greater than 100% yield, this is probably due to there still being copper oxide present alongside the copper. Alternatively, students can remove the copper from the tube prior to weighing it and attempt to separate out the copper from the copper oxide. This gives a lower yield (neither is particularly accurate).
Related resources
- Students can find the formula of copper(II) oxide based on its reduction by methane in a related class practical.
- Try another microscale experiment, illustrating the cracking of hydrocarbons using paraffin, bromine water and aluminium oxide.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet